wavelength-dispersive X-ray spectrometer, WDS, WDX
wavelength-dispersive X-ray spectrometer
As shown in Fig. 1, the spectrometer is composed of an analyzing crystal and an X-ray detector. The X-ray generation point on the specimen, the analyzing crystal and the X-ray detector are set on the circumference of a circle called Rowland circle. Those three are placed so that the distance between the X-ray generation point and the analyzing crystal is always the same as the distance between the analyzing crystal and the X-ray detector. Owing to this arrangement, the characteristic X-rays emitted from the generation point are collected on a point of the detector, where only the characteristic X-rays which satisfy Bragg’s law (λl = 2dsinθ) are reflected (diffracted) by the analyzing crystal and reach the X-ray detector. To detect characteristic X-rays with a different wavelength, a different Bragg reflection is used by moving the analyzing crystal to change the incidence angle θ of the X-ray onto the analyzing crystal, where the X-ray detector is moved on the circumference of the Rowland circle in conjunction with the movement of the analyzing crystal. It should be noted that the Rowland circle itself also moves along with the movement of the analyzing crystal. The analyzing crystal is moved along a straight line (in Fig. 1) to keep the take-off angle (φ in Fig. 1) constant, which makes the condition of X-ray absorption in the specimen (path length) same for different characteristic X-rays. Fig. 2 shows two types of analyzing crystals. The Johann type crystal is designed for an efficient collection of the X-rays in such a way that a crystal is curved with a curvature of two times larger than that of the Rowland circle. The Johansson type crystal is created for a high precision collection of the X-rays in such a way that the surface of a Johann type crystal is polished so as to fit the crystal to the Rowland circle.
To acquire characteristic X-ray spectra over a wide wavelength range, the spectrometer system incorporates a plural number of analyzing crystals with different lattice spacings. These analyzing crystals are used properly according to the wavelength range to be analyzed. Typical analyzing crystals are as follows. 1) Small lattice spacing: lithium fluoride (LiF(200), spacing: 0.4 nm), 2) Medium lattice spacing: pentaerythritol (PET(002), spacing: 0.87 nm), and 3) Large spacing: thallium acid phthalate (TAP(100), spacing: 2.6 nm) and stearate (STE, spacing: 10 nm). STE is called a super-lattice X-ray analyzing crystal and is a laminated film of fatty-acid-lead, and the arrangement of lead atoms on the fatty acid behave as crystalline lattice planes.
The table below lists the analyzing crystals and their target elements.
| Analyzing crystal | Element (K-line) | Element (L-line) | Element (M-line) |
|---|---|---|---|
| TAP | O to P | Cr to Nb | La to Hg |
| STE | B to O | Ca to Cr | |
| PET | Si to Ti | Pb to La | Ta to U |
| LiF | Ca to Rb | Sn to U |
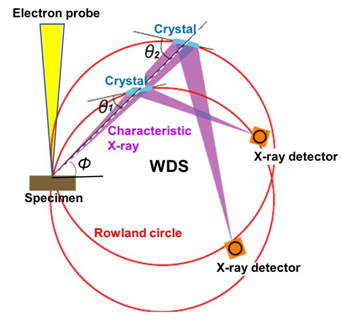
Fig. 1 Principle of WDS
WDS spectrometer is designed so that the specimen (analysis point), analyzing crystal and X-ray detector are always situated on the circumference of the Rowland circle. To detect a characteristic X-ray with a different wavelength, the analyzing crystal is moved so as to change the incidence angle θ of the X-ray onto the analyzing crystal for using a different Bragg reflection. To acquire characteristic X-ray spectra over a wide wavelength range, a plural number of analyzing crystals with different lattice spacings are used. A spectrometer, in which the analyzing crystal is moved along a straight line to keep the take-off angle φ constant, is called a WDS spectrometer with linear crystal motion. This type of spectrometer is adopted in WDS for an SEM instrument.
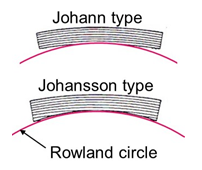
Fig. 2 Shape of analyzing crystal
The Johann type crystal (top in the figure) is designed in such a way that a crystal is curved up to the curvature being two times larger than that of the Rowland circle. The Johansson type crystal (bottom in the figure) is designed in such a way that the crystal is polished and curved up to the curvature being the same as that of the Rowland circle.
Related Term(s)
Term(s) with "wavelength-dispersive X-ray spectrometer" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.




