Exciton
Exciton
Consider a state in which an electron in a solid is excited and a hole is created where the electron was occupied by a light beam or an electron beam. This hole is positively charged. The excited electron and the created hole are attracted to each other by the Coulomb force, forming an electron-hole pair. This is called the exciton. The hole and electron forming the exciton make a circular orbital in the solid while maintaining a certain distance from each other. The exciton forms an energy level with a narrow energy width at an energy position of tens to hundreds of meV below the lowest energy level of the conduction band. This energy level is observed as a sharp peak near the onset energy position of an electron energy-loss (EELS) spectrum. Fig. 1 (a) shows a core-loss spectrum of diamond. This spectrum corresponds to the transition from the 1s core-level to the conduction band of diamond, and exhibits the density of states (DOS) distribution of the conduction band. A sharp peak observed at 289 eV (▼) is the exciton peak.
Fig. 1 (b) shows the schematic of the electronic structure of diamond. The exciton is formed by the hole created in the 1s core-level and the excited electron. In this case, the size (diameter) of the exciton is around 0.1 nm. The energy level of the exciton of diamond is formed at about 0.2 eV below the lowest energy level of the conduction band (indicated by a red dotted line in Fig. 1 (b) ).
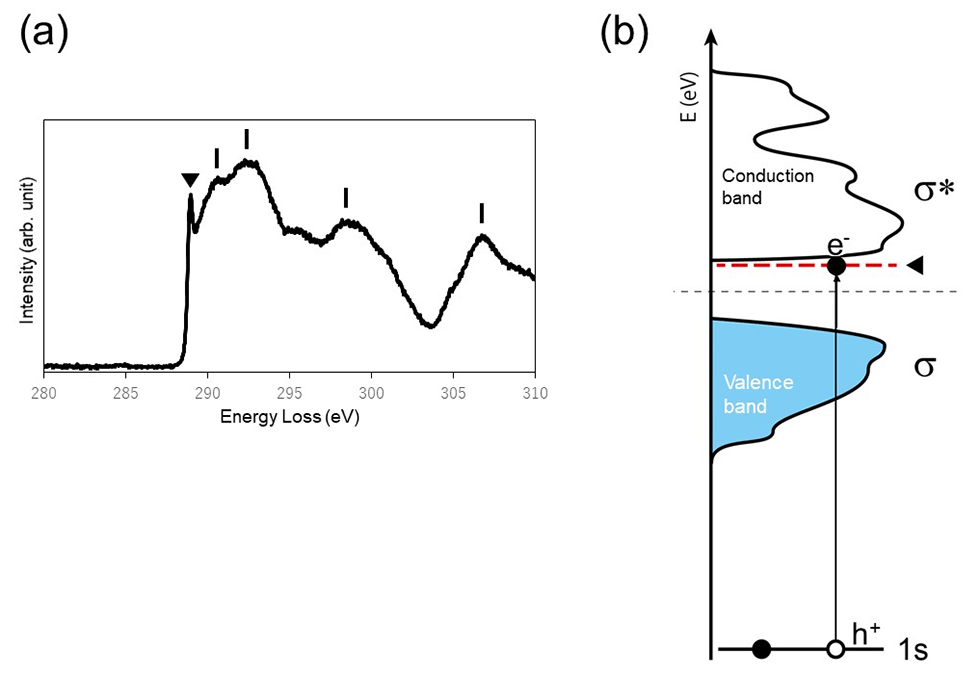
Fig. 1(a) Core-loss spectrum of diamond. A sharp peak at 289 eV (▼) is the exciton peak. The spectral intensity distribution extending from the exciton peak at the high energy side exhibits the DOS of the conduction band. (b) Schematic of the electronic structure of diamond. The electron beam excites an electron (e-) in the 1s core-orbital to create the hole (h+) in the 1s orbital. The excited electron is attracted to the hole with the positive charge, forming the exciton level (red dotted line) at about 0.2 eV below the lowest energy level of the conduction band of diamond.
Fig. 2(a) shows a valence-loss spectrum of SiO2. The onset of the spectrum observed near the band gap energy (~9 eV) of SiO2 corresponds to the transition from the highest energy level in the valence band to the lowest energy level in the conduction band. A spectral peak (▼) in Fig. 2(a)) observed just above the onset energy is the exciton peak. The schematic of the electronic structure of SiO2 is shown in Fig. 2(b). The exciton is formed by the hole created in the valence band and the excited electron. The exciton in this case spatially is spread to a size (diameter) of a few nm in diameter because the Coulomb force is weaker than the case of Fig. 1. Then, the energy level of the exciton (shown by pink in Fig. 2(b)) is formed at the bottom of the conduction band. In addition, the exciton in SiO2 can move in the range of tens to hundreds of nm by diffusion and can recombine and disappear during diffusion.
It is important to note that the valence-loss spectrum of Fig. 2(a) is the loss function but not the imaginary part of the dielectric function as shown in Fig. 1(a). Thus, the accurate value of the energy of exciton has to be obtained from the peak energy of the imaginary part of the dielectric function by deriving the dielectric function from the loss function using Kramers-Kroning analysis. However, the exciton peak of SiO2 observed in the loss function is sufficiently sharp and almost identical to the energy position appearing in the imaginary part of the dielectric function.
(By Associate Professor Yohei Sato, Tohoku University)
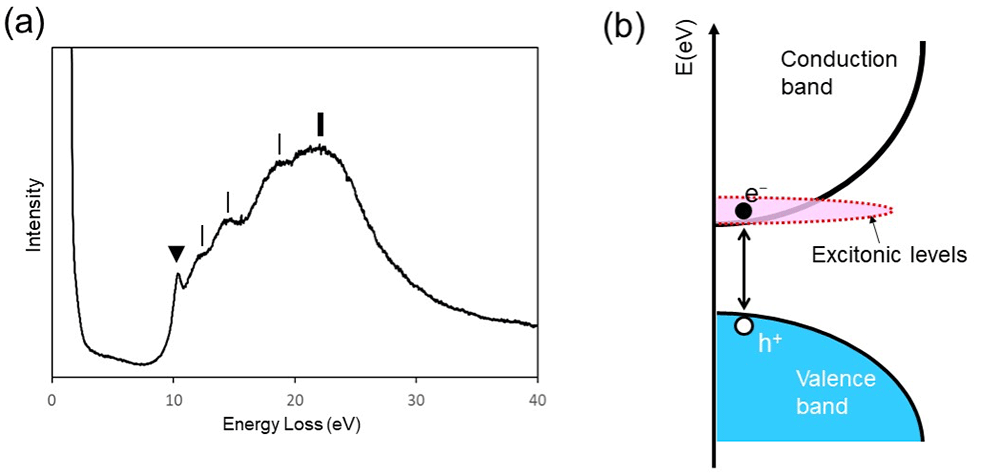
Fig. 2(a) Valence-loss spectrum of SiO2. A spectral peak at 10.4 eV (▼) is the exciton peak. A peak at 22 eV (bold vertical line) is the volume plasmon peak due to collective oscillations of the whole valence electrons of SiO2. The other spectral structures (fine vertical lines) are originated from interband transitions. (b) Schematic of the electronic structure of SiO2. The excited electron (e-) is bounded weakly with the hole (h+) created in the valence band. As a result, the energy level of the exciton (shown by pink in (b)) is formed at the bottom of the conduction band of SiO2.
Related Term(s)
Term(s) with "Exciton" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.