zeta factor method, ζ factor method
zeta factor method, ζ factor method
A quantitative elemental analysis method using energy-dispersive X-ray spectroscopy (EDS) in a TEM [1]. The ζ (zeta) factor method improves the Cliff-Lorimer method which has widely been used for EDS quantitative analysis. The main advantage of the ζ factor method exists in high-accuracy quantitative analysis even when the density and thickness of a specimen are unknown.
Chemical (elemental) composition determination using the characteristic X-ray
In the ζ (zeta) factor method, the chemical (elemental) composition of the specimen is determined by multiplying the factor ζi to the measured characteristic X-ray intensity Ii using the following equation.
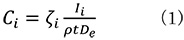
Ci: Chemical composition of Element i
Ii: Characteristic X-ray intensity of Element i
ρt:Mass thickness
De: Electron illumination dose (De = Electron charge Χ Illumination current Χ Measurement time)
The methods of obtaining ζi and ρt used in the equation (1) are described in the following way.
Determination of the ζ (zeta) factor
The ζ (zeta) factor (ζi) of element i is defined by using ionization cross section Qi, fluorescence yield ωi (characteristic X-ray generation probability) and electron illumination dose De.
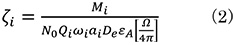
M: Atomic weight
N0: Avogadro constant
Qi: Ionization cross section of the characteristic X-ray from Element i
ωi: Fluorescence yield of the characteristic X-ray from Element i
ai: Ratio of Kα (Lα or Mα) lines to all of K (L or M) lines from Element i
De: Electron illumination dose (De = Electron charge Χ Illumination current Χ Measurement time)
εi : Detection efficiency of the characteristic X-ray from Element i
Ω: Detection solid angle
The ζ (zeta) factor can be determined theoretically by the equation (2). However, since the value of Qi is dependent on the model to obtain the ionization cross-section, the value of ζ factor is somewhat arbitrary. Thus, in many cases, the ζ factor is experimentally determined using a thin film standard specimen, which contains the target element and has a known composition and thickness.
Determination of mass thickness (ρt)
In the ζ (zeta) factor method, the mass thickness (ρt) of the specimen can be estimated using the measured characteristic X-ray intensity as expressed below.
Taking the sum for the compositions of all constituent elements, ∑i Ci =1. Then, the equation (1) is expressed as follows.
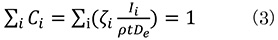
From equation (3), the mass thickness (ρt) is obtained by the next equation.
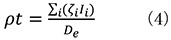
Therefore, the ζ (zeta) factor method offers determination of the chemical (elemental) composition of the specimen from the measured characteristic X-ray intensity using the ζ factor obtained through the equation (2) or experimentally, with the mass thickness obtained by the equation (3), even when the density and thickness of the specimen are unknown. It should be noted that the method enables high-accuracy quantitative analysis even for the characteristic X-rays with low energies (soft X-rays) where the absorption effect in the specimen is negligible.
The Cliff-Lorimer method is used commonly for the EDS quantitative analysis. However, by this method, proper correction of the absorption effect of characteristic X-rays can be performed only when the density and thickness of a specimen are known. Thus, the accuracy of the quantitative analysis of EDS is not sufficient for the characteristic X-rays, which suffers the absorption effect as far as the density and thickness of the specimen are unknown.
[1] M. Watanabe and D.B. Williams: "The Quantitative Analysis of Thin Specimens: a Review of Progress from the Cliff-Lorimer to the New ζ-Factor Methods", J. Microsc. 221 (2006), 89-109.
(By Professor Masashi Watanabe, Lehigh University)
Related Term(s)
Term(s) with "zeta factor method, ζ factor method" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.