characteristic X-ray
characteristic X-ray
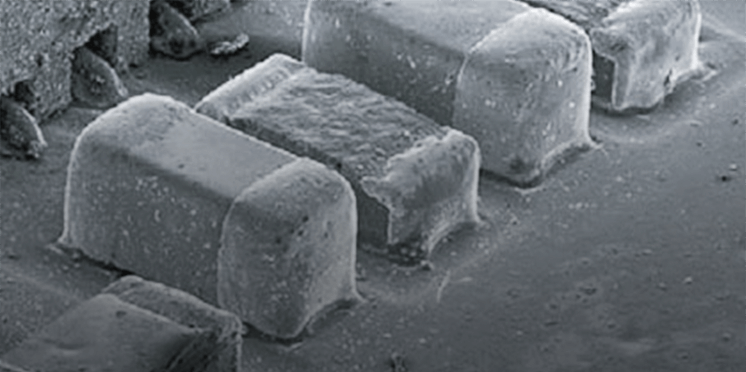
When one of inner-shell electrons is excited, an electron vacancy is formed in the inner-shell states. Then, an outer-shell electron, whose energy level is higher than that of the inner-shell electron, falls on the vacancy while emitting X-rays. The energy of the emitted X-rays corresponds to the energy difference between the outer-shell electron and the inner-shell electron. The emitted X-rays are called "characteristic X-ray(s)" and are characteristic of individual atoms. The characteristic X-rays are utilized for qualitative and quantitative element analysis on a micro-area.
Related Term(s)
Term(s) with "characteristic X-ray" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.