scattering cross section
scattering cross section
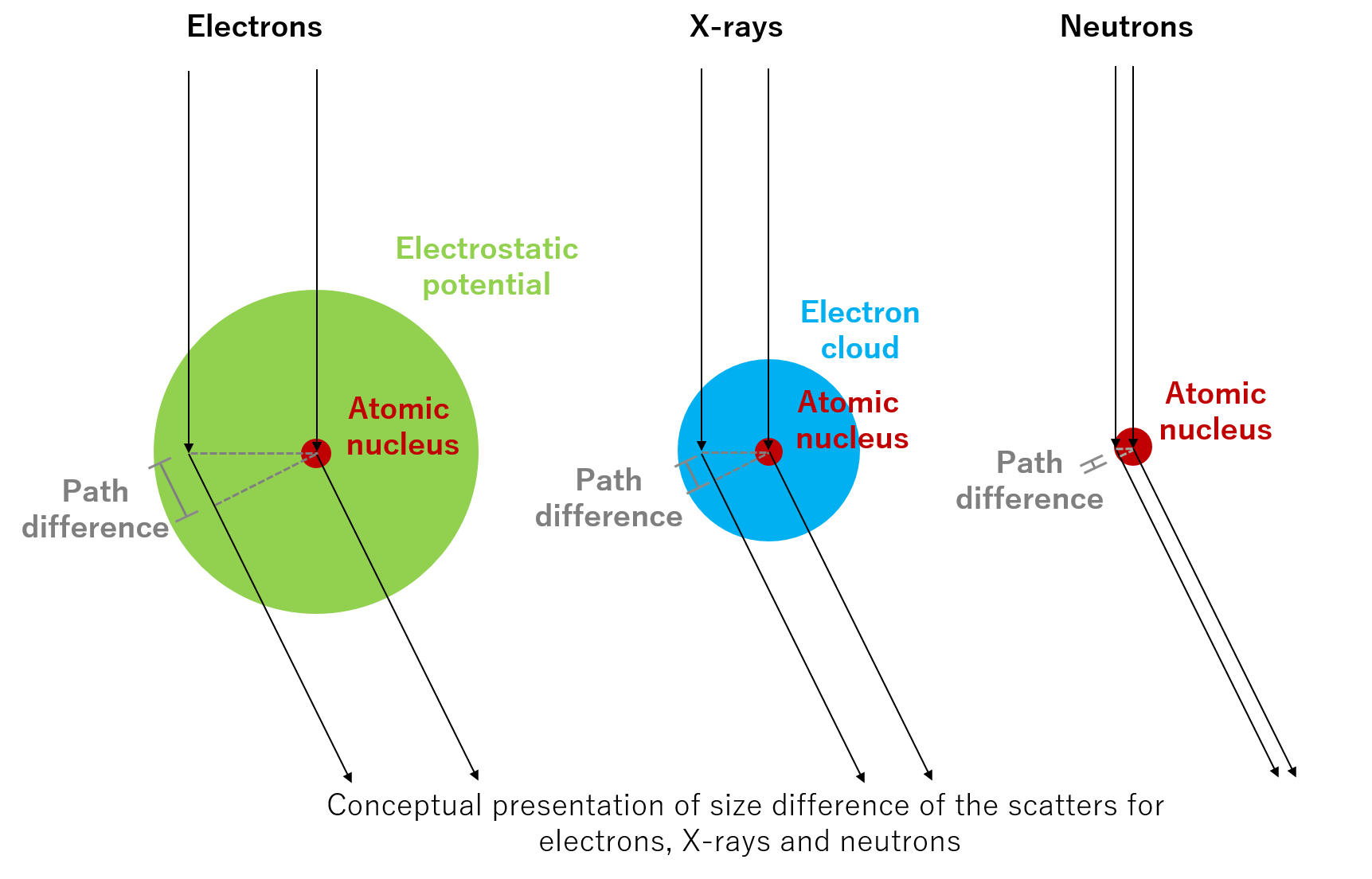
The scattering cross section is the scattering probability of an incident electron being scattered by an atom, expressed by the unit of area.
The relation between the atomic scattering factor (atomic form factor) and the scattering cross section for incident electrons is as follows.
The atomic scattering factor for an incident electron (in units of [V][Å3]) multiplied by 2πme/h2=2.09x10-2 ([V¯1][ů2]) is the scattering length ([Å]). The square of the scattering length is the scattering cross section ([Å2]).
Since the atom size is finite, the scattering cross section is large at low scattering angles because the phase difference between the electrons scattered by the inner part and outer part of the atom is small. As scattering angles become large, the cross section becomes small because the phase difference between those electrons becomes large, and thus the interference of those electron waves is destructive.
It is noted that 2πme/h2 is the coefficient that appears when solving scattering problem using the Schrödinger equation. Here, m is the mass of an electron, e is the charge of an electron, and h is Planck’s constant.
Related Term(s)
Term(s) with "scattering cross section" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.