Case study of samples containing phenylphosphine
NM240009E
Numerous phosphorus-containing compounds with Phenylphosphine structures are important catalysts. The 13C signals of carbon atoms near phosphorus atoms are split by 13C31P scalar couplings. In particular, structures containing multiple phenyl groups pose challenges, as carbon signals in these phenyl groups are detected in a narrow chemical shift range, making spectral analysis very difficult. Therefore, here we present measurement examples that are useful for signal assignment of such structures. We utilized the ROYALPROBETM P+[1], enabling1H, 31P, and X triple-resonance experiments in conjunction with the standard JNM-ECZL600G configuration. Notably, even with the standard 2-channel NMR system in the JNM-ECZL series, it is possible to generate frequencies of three nuclei in a single experiment [2]
Preliminary experiment
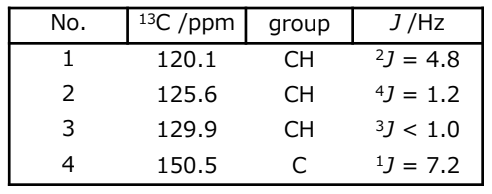
First of all, let’s focus on 48.5 mM triphenylphosphine with a phenylphosphine structure. This sample has three equivalent phenyl groups, and hence NMR detects only one type of phenyl ring. Fig. 1 shows the 13C NMR spectrum collected with proton decoupling. The carbon signals are numbered from the high field side to the low field side. Additionally, Fig. 1 depicts the expansions of each carbon signal. It is clear that the four signals are not singlets – they are all split into doublets by 13C-31P couplings, including one-bond, two-bond, three-bond, and four-bond couplings. The molecular structure of triphenylphosphine and the numbering of carbon atoms are depicted in Fig. 2, which provides context for understanding the NMR data. Table 1 summarizes the 13C chemical shifts and 13C-31P coupling constants. This result demonstrates that even direct 13C-31P coupling is not large coupling constant.
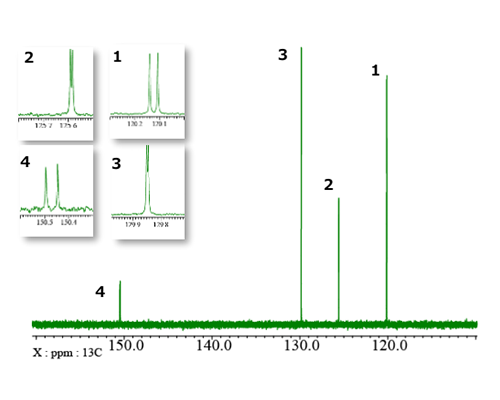
Fig. 1: 13C{1H} spectrum
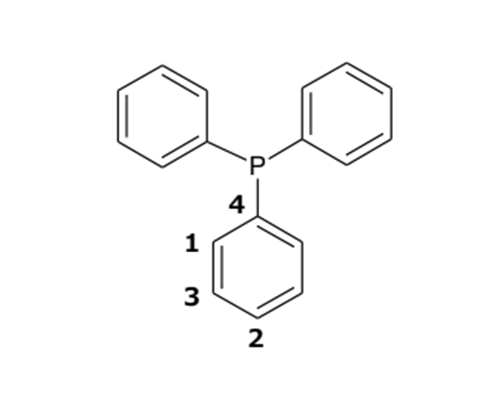
Fig. 2: Structural formula of triphenylphosphine and atom numbering
Table 1: 13C chemical shifts and coupling constants
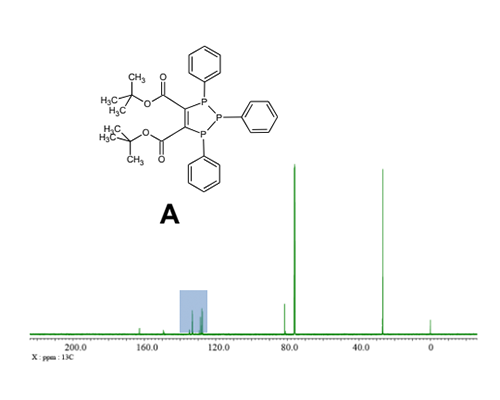
13C NMR spectrum of compound with non-equivalent phenyl groups
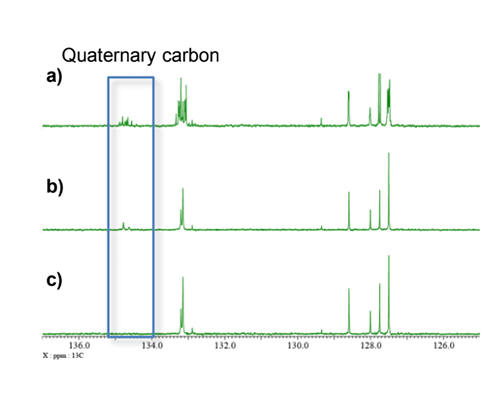
The 13C spectra of a sample prepared by dissolving 10 mg of di-t-butyl 1,2,3-triphenyl-2,3-dihydro-1H-1,2,3-triphosphole-4,5-dicarboxylate A [3] in CDCl3 are shown in Fig. 3. While the compound comprises three phenyl rings, only two of them are equivalent. Consequently, two sets of 13C signals representing the aromatic rings are detected. Due to the splitting of aromatic carbon signals by 13C-31P couplings, the 13C{1H} spectrum appears highly congested in the aromatic region, posing challenges for analysis. Fig. 4 displays the aromatic range of the 13C{1H} spectrum and provides a comparison with the 13C{1H}{31P} and DEPT{1H}{31P} triple-resonance spectra. The simultaneous 1H and 31P decoupling simplifies the spectral analyses significantly. Moreover, the DEPT experiment suppresses the signals of quaternary carbons, tertiary carbons can be easily distinguished.
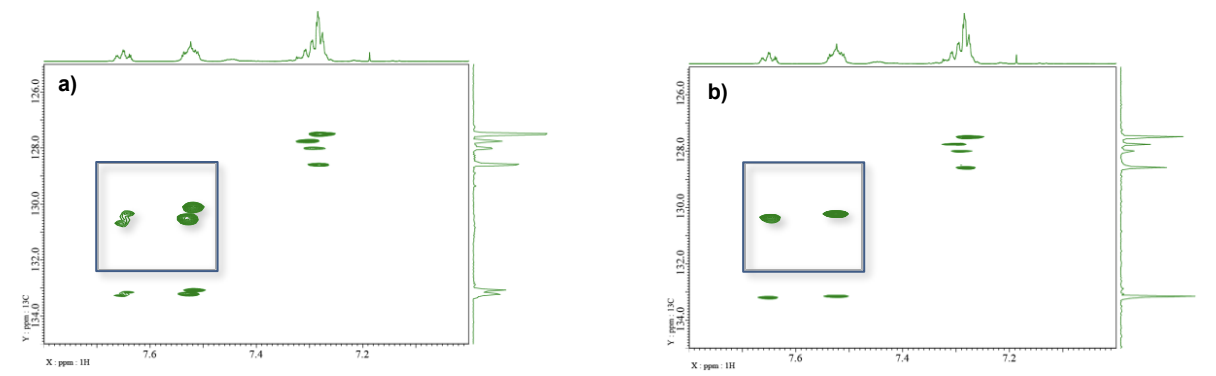
Adding 31P decoupling to 1H-13C HSQC
Fig. 5 presents the 1H-13C HSQC spectra of sample A without (a) and with 31P decoupling (b). Due to the small coupling between carbon and phosphorus, this effect may not be easily discernible in standard 2D NMR spectra. However, in cases where spectra are crowded, resolution enhancement techniques become necessary. Under such conditions, the beneficial effect of 31P decoupling becomes more apparent, aiding in the clearer interpretation of the spectra.
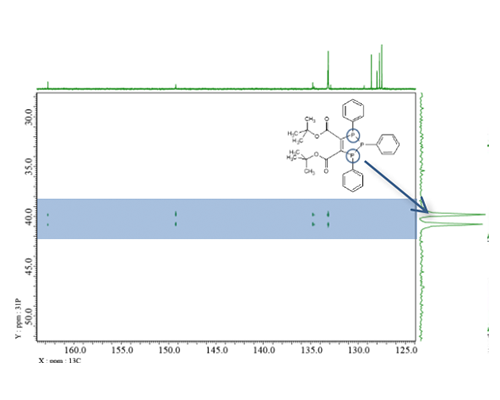
13C-31P correlation measurement using J Cross Polarization
I have shown that the 13C chemical shifts are crowded in the aromatic region and 1JCP and nJCP coupling constants do not change significantly in phenylphosphine structure. Here is an example of 2D measurement using J Cross Polarization (JCP) for such a sample. JCP also called HETERO TOCSY or HEHAHA, is a method of transferring magnetization to different nuclei like INEPT[4]. JCP condition (Hartmann-Hahn condition) of solution, which is difficult to achieve excitation of all 13C signals, so it is generally not used in comparison to INEPT. However, INEPT is anti-phase magnetization transfer, so decoupling cannot be performed immediately after the magnetization transfer. On the other hands, JCP is in-phase magnetization transfer, so decoupling is possible immediately after the magnetization transfer even in long-distance correlations. Therefore, decoupling spectra with simplified signal patterns can be obtained. In other words, this method is useful for this sample, which requires a high resolution spectrum with a narrow chemical shift range required for 13C. On the other hands, for samples such as alkyl phosphonates, where the coupling constants differ greatly between 1JCP and nJCP, the efficiency of magnetization transfer to small couplings is reduced, this method inappropriate if you want to observe even long-distance correlations. Fig. 6 shows the 13C-31P JCP spectrum, and Fig. 7 compares the 13C and JCP slice spectra. Fig. 6 shows that 13C signals coupled with a specific 31P signal can be observed with high resolution. Also, as shown in Fig. 7 c), as same as “mixing time” of TOCSY, small coupling correlations are also easier to observe by increasing the jcp time .
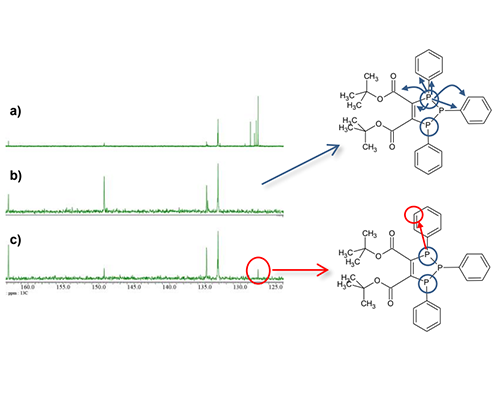
a) 31C{1H}{31P} spectrum
b) 13C- 31P JCP {1H}{31P} spectrum, jcp_time : 33.5ms
c) 13C- 31P JCP {1H}{31P} spectrum, jcp_time : 67ms
Sample courtesy of
Prof. Mieko Arisawa, Asst. Prof. Yasutaka Kawai
(Faculty of Agriculture, Kyushu University)
Reference :
: [1] JEOL Application note NM220010
: [2] JEOL Application note NM220004
: [3] Arisawa, M.; Otsuka, H.; Idogawa, T.; Sawahata, K.; Kawai, Y. Asian J. Org. Chem., 2024, in press.
: [4] RSC Adv., 2022, 12, 10062-10070
Solutions by field
Related products
Related information
New function of ECZ Luminous part 1- Multiple Frequency Drive System
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

