Application of ROYALPROBE™ P+ to catalyst
NM220010E
Feature of ROYALPROBE™ P+
The ROYALPROBE™ P+ is a newly developed solution NMR triple-resonance probe that is capable of 1H, X, and 31P triple-resonance measurements. It can be used as a triple-resonance probe even on a standard 2-channel ECZ Luminous console due to the MFDS function1) . Fig. 1 is a wiring diagram of the ROYALPROBE™ P+ connected to a 2-channel ECZ Luminous console. In the case of conventional triple-resonance probe, it is necessary to manually exchange the corresponding frequency filter every time the X nucleus is changed, therefore it is not possible to change the X nucleus by AUTO TUNING UNIT only. In contrast, the ROYALPROBE™ P+ uses a newly developed PX diplexer that can serve as a filter for various X nuclei. This allows us to tune the probe on all three channels automatically using the AUTO TUNING UNIT. Furthermore, compared to a conventional triple-resonance probe, the sensitivity of 13C, 31P and other nuclei has been greatly improved, making it much more versatile. Here we demonstrate an application of this new probe to a sample of catalyst containing 31P.
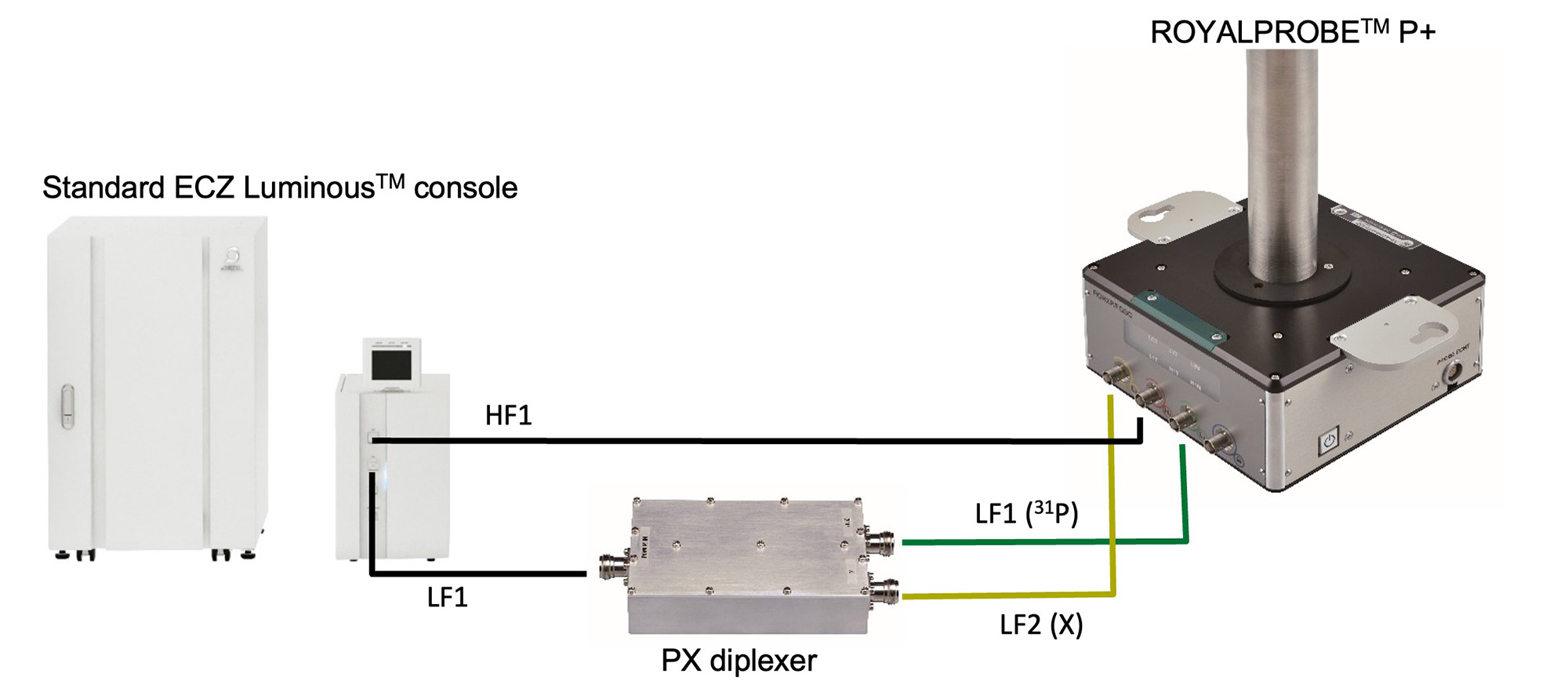
Fig. 1: Wiring of ROYALPROBE™ P+ and HPX filter when using ECZ Luminous in standard configuration
Triple-resonance experiment of 1H, 11B and 31P
For many organic compounds, the 1H chemical shift range is about 10 ppm, but it is known that the 1H chemical shift may be significantly shifted by metals. Fig. 2 shows a 1H NMR spectrum of Ru-MACHO®-BH ※ which is known as an ester hydrogenation catalyst. A very characteristic 1H signal can be observed in high field above -10 ppm which is a very unusual value. . In addition, a very broad and split signal at -2.3 ppm is observed. Since this sample contains heteroatoms such as 31P and 11B that affect the shape of 1H signal, decoupling of these nuclides can help us identify the 1H nuclei close to the phosphorus and boron atoms.
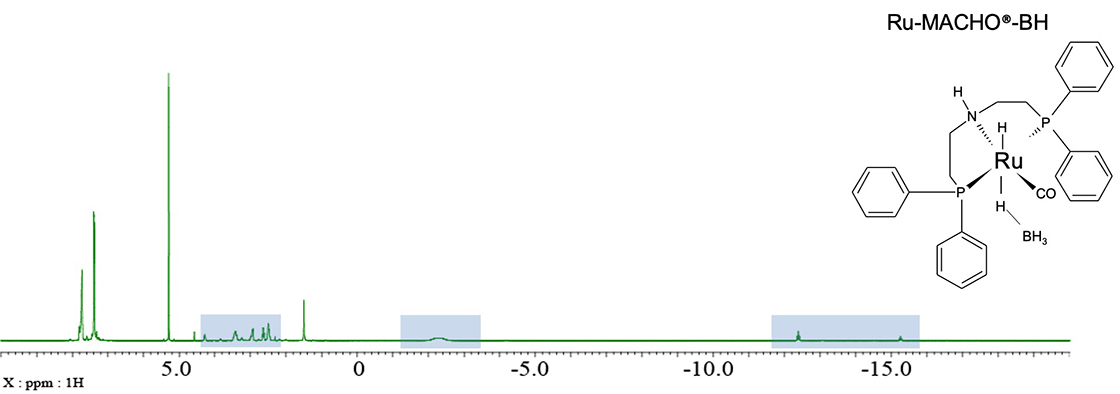
Fig. 2: 1H NMR spectrum of 3mg Ru-MACHO®-BH in CD2Cl2
※Ru-MACHO® is a registered trademark of TAKASAGO INTERNATIONAL CORPORATION.
The spectrum shown in Fig. 2 is further expanded in Fig. 3-5. The CH2 range is shown in Fig. 3. The signal of BH4 group is shown in Fig. 4. Finally, the signals of Ru-H protons are shown in Fig. 5. a) refers to standard 1H spectra, b) refers to 1H{11B} spectra, c) refers to 1H{31P} spectra, and d) refers to 1H{11B}{31P} spectra. By careful comparison of the coupled and uncoupled 1H spectra, it is possible to identify the signals of the CH2 and Ru-H groups and the signal of the BH4 group. In catalysts containing transition metals, such as this sample, it is common for various stereoisomers to exist and be present. For this reason, more signals are often observed than one would expect from the structural formula. This phenomenon can also be confirmed in Fig. 3-5 where two sets of signals are clearly observed. Therefore, narrowing and simplifying signals by heteronuclear decoupling may help reduce overlaps and identify minor components and/or impurities.
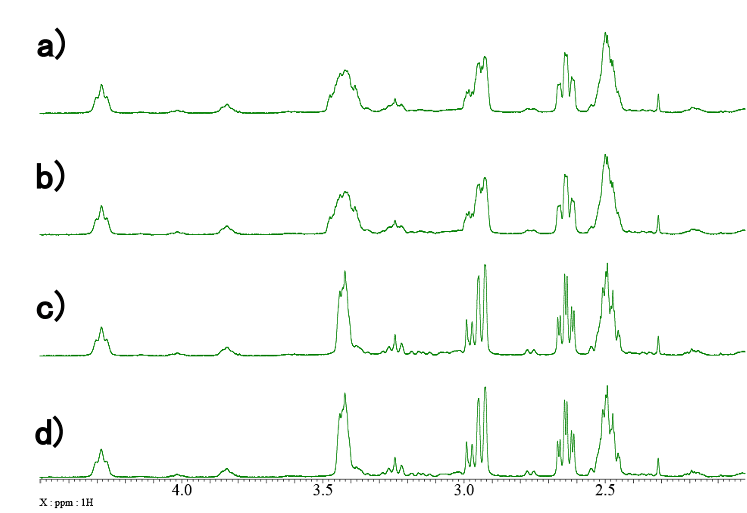
Fig. 3: Expanded region of CH2 groups
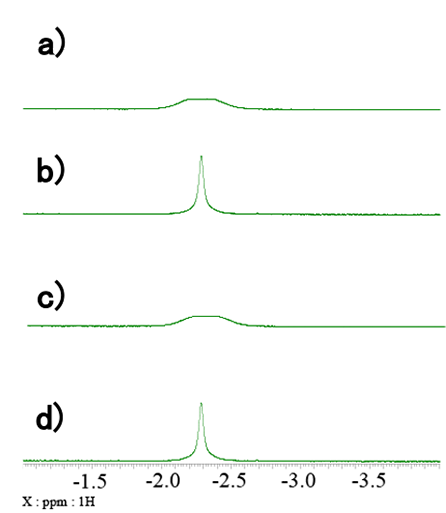
Fig. 4: Expanded region of BH4 group
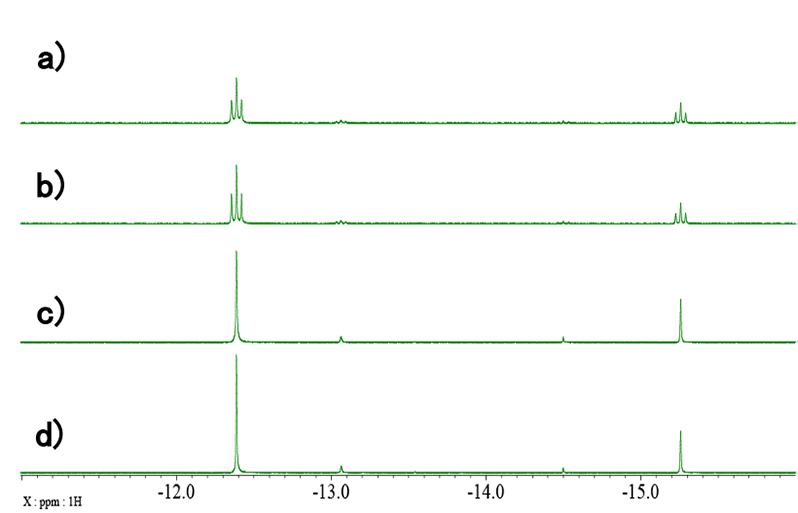
Fig. 5: Expanded region of Ru-H group
Triple-resonance experiment of 13C, 1H and 31P
Fig. 6 shows a standard 13C{1H} spectrum of the catalyst, whilst Fig. 7 shows the expansion indicated by the blue rectangle in Fig. 6. As you can see in the 13C{1H}{31P} spectrum (Fig 7b), the signals of CH groups and quaternary carbons in the vicinity of 31P are very easy to distinguish by comparing the double-resonance and triple-resonance spectra. As 13C is a low sensitivity nuclide due to its low natural abundance (1.1%), the signals of carbon atoms coupled with 31P may be difficult to detect in the case of diluted samples like this one. For this reason, 31P-decoupling simplifies the spectrum and allows us to observe signals which could be probably missed.
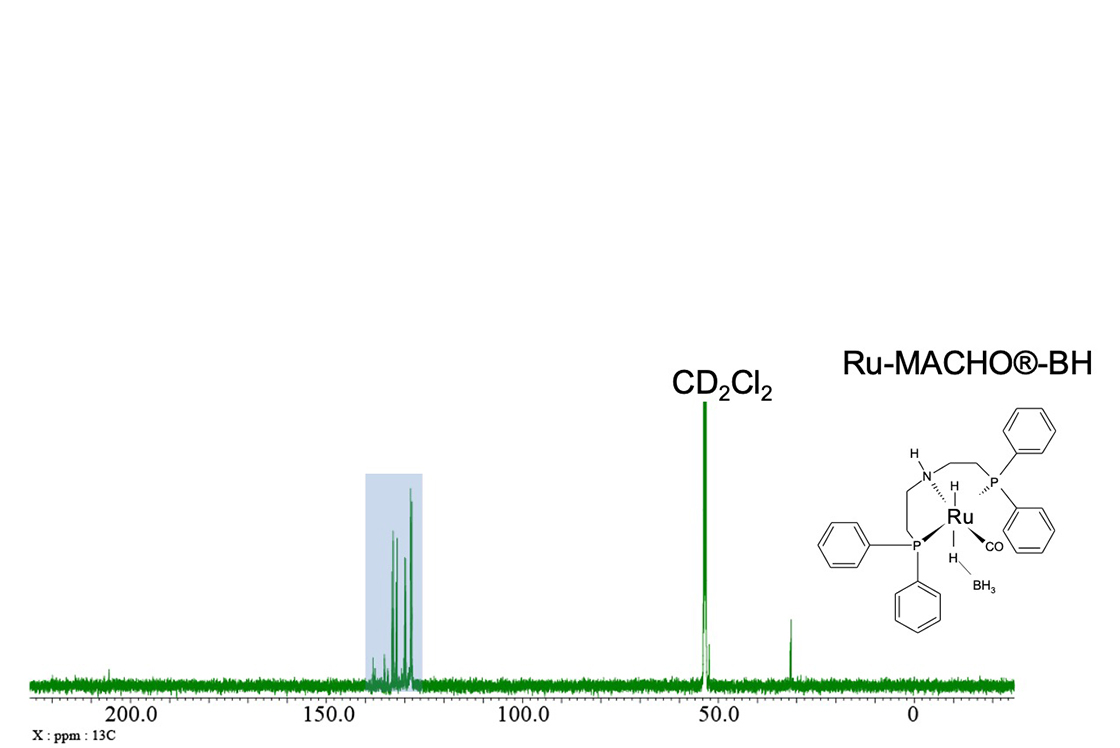
Fig. 6: 13C{1H} spectrum
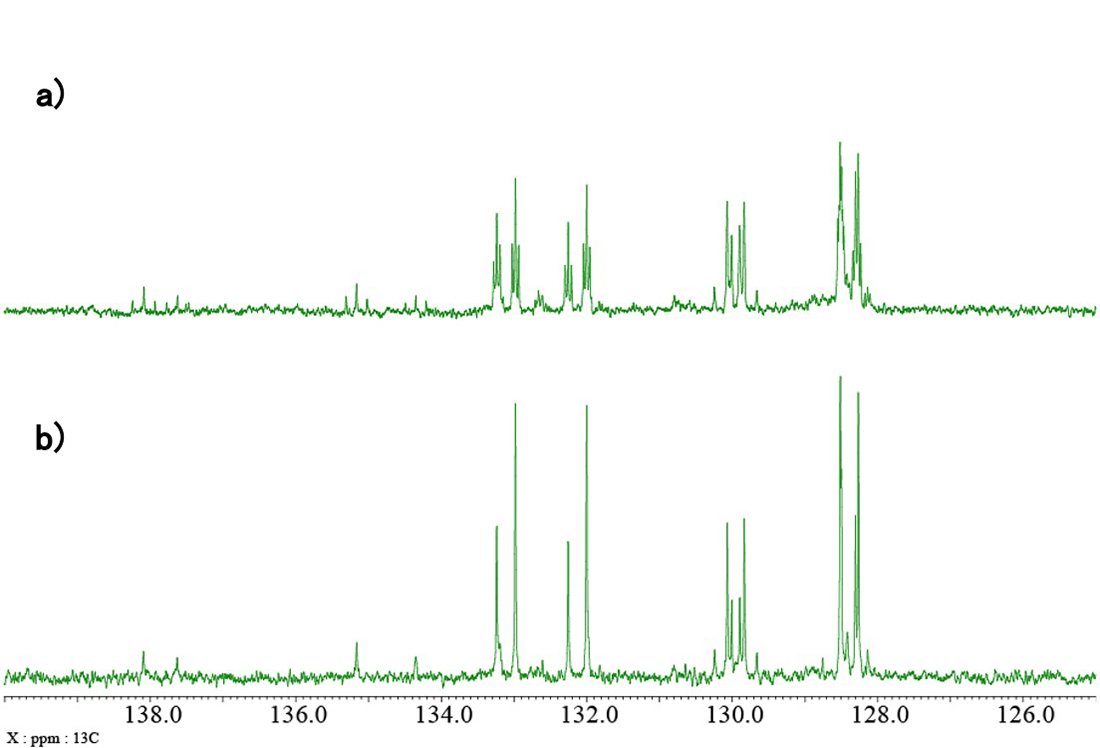
Fig. 7: Expanded aromatic region of a) 13C{1H}, b) 13C{1H}{31P} spectra
In the case of samples containing stereoisomers or multiple components, complex spectral patterns are often observed. Therefore, the capability to decouple 31P and other heteronuclei and to employ advanced correlation experiments is very important in the analysis of complex samples. Therefore, the ROYALPROBE™ P+ and other triple-resonance probes utilizing the MFDS functionality of the ECZ Luminous are of great help to those who synthetize and analyze such samples.
Reference
Sample courtesy of TAKASAGO INTERNATIONAL CORPORATION
Solutions by field
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
