Residual solvent analysis in cosmetic ingredient using single QMS mode and alternative carrier gases in GC/MS/MS
MSTips No. 466
Introduction

Fig. 1 JMS-TQ4000GC UltraQuad™ TQ
The gas chromatograph triple quadrupole mass spectrometer JMS-TQ4000GC UltraQuad™ TQ (Fig. 1) can be used as QMS by single QMS mode in addition to qualitative and quantitative analysis of trace components by MS/MS mode. We have already reported quantitative analysis in water samples and qualitative analysis in material samples in MSTips No. 443 and 450. In this time, we report the analysis of residual solvents in cosmetic ingredient using the single QMS mode. Residual solvents in cosmetic ingredient, like residual solvents in pharmaceuticals, are classified as Class 1-3 solvents by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Class 1 solvents: Solvents to be avoided in the manufacture of pharmaceuticals. Class 2 solvents: Solvents to be limited in pharmaceuticals. Class 3 solvents: Solvents with low toxic potential[1]. In this application note, we report the analysis of residual solvents in ethanol solution using nitrogen gas as an alternative carrier gas for seven solvent components classified as Class 1 and 2, as well as the quantitative analysis of 1,4-Dioxane in cosmetic ingredient.
Method
The measurement was performed using a MS-62071STRAP trap-type HS and GC triple quadrupole mass spectrometer JMS-TQ4000GC UltraQuad™ TQ. The measurement conditions are shown in Table 1, and the target components and their SIM monitoring ion are shown in Table 2.
Table 1 Measurement condition
| Sample | |
|---|---|
| Sample 1 | Standard 7 mix in EtOH (Mixture of Methyl alcohol, Methylene chloride, Trichloromethane, Benzene, 1,4-Dioxane, Toluene and Xylene including Ethylbenzene. Concentration levels for each compound range from 0.2-100 ppm.) |
| Sample 2 | Cosmetic ingredient including 1,4-Dioxane at about 1 ppm |
| HS condition | |
| Sample Volume | Sample 1: 2 μL, Sample 2: about 1g with 1,4-Dioxane 4 μL and d8-Dioxane 4 μL |
| Sample Temp. | Sample 1: 100°C, Sample 2: 80°C |
| Sampling mode | Loop mode |
| Heating time | Sample 1: 60 min, Sample 2: 30 min |
| GC condition | |
| Column | Rxi-624 (30 m length, 0.25 mm i.d., 1.4 μm film thickness) |
| Inlet | Split/Splitless |
| Inlet Temp. | 200°C |
| Flow | He carrier gas 1 mL/min, Constant flow N2 carrier gas 1 mL/min, Constant flow |
| Injection Mode | Split (50 :1) |
| Oven Program | 35°C (5 min) → 10°C/min → 250°C (5 min) |
| MS condition | |
| Ion Source Temp. | 200°C |
| Interface Temp. | 200°C |
| Ionization Mode | EI+, 70 eV (He carrier gas), 20 eV (N2 carrier gas) |
| Measurement Mode | SIM |
Table 2 Monitoring ion of SIM
| Component | Quantitative ion | Reference ion 1 | Reference ion 2 |
|---|---|---|---|
| Methyl Alcohol | 31 | 29 | 32 |
| Methylene chloride | 84 | 49 | 86 |
| Trichloromethane | 83 | 85 | 118 |
| Benzene | 78 | 77 | 52 |
| d8-Dioxane | 96 | 64 | 46 |
| 1,4-Dioxane | 88 | 58 | 43 |
| Toluene | 91 | 92 | 65 |
| Ethylbenzene | 91 | 106 | 65 |
| m,p-Xylene | 91 | 106 | 65 |
| o-Xylene | 91 | 106 | 65 |
Result
⚫︎Measurement result of sample 1
The observed EIC peaks of each component by He carrier gas and N2 carrier gas are shown in Fig. 2. All target components were observed by both carrier gases. On the other hand, when focusing on methyl alcohol, the EIC peak of m/z 29 is buried in the baseline for the N2 carrier gas. It is supposed that baseline was higher than that of the He carrier gas by stable isotope(15N) of N2. The comparison result of S/N value is shown in Table 3. Generally, it is said that the sensitivity of N2 carrier gas is about 1/10 lower than that of He carrier gas. However, for this measurement, the S/N values of 8 out of 9 components were within 1/5. It is assumed that the ionization energy of 20 eV suppressed the generation of nitrogen ion, which is a factor in sensitivity reduction, and as a result, good sensitivity was obtained.
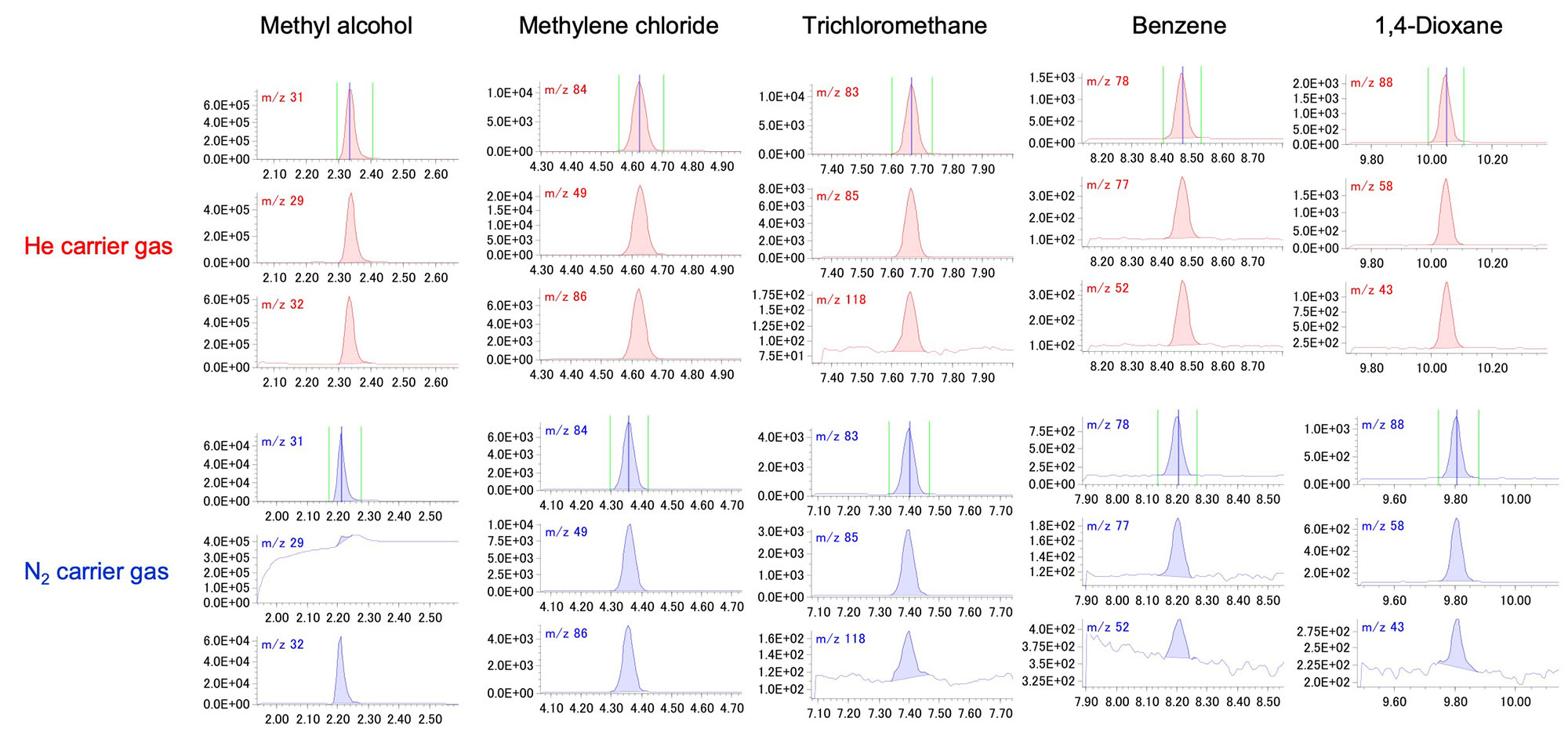
Fig. 2 The observed EIC peaks of each component by He carrier gas and N2 carrier gas
Table 3 Comparison S/N value by He carrier gas with S/N value by N2 carrier gas
| Component | S/N value by He carrier gas | S/N value by N2 carrier gas |
|---|---|---|
| Methyl Alcohol | 105022.7 | 24079.0 |
| Methylene chloride | 14312.2 | 10044.2 |
| Trichloromethane | 7131.1 | 5385.4 |
| Benzene | 2288.3 | 1065.5 |
| 1,4-Dioxane | 3098.7 | 913.9 |
| Toluene | 1365053.9 | 256462.1 |
| Ethylbenzene | 33704.9 | 4821.1 |
| m,p-Xylene | 99506.4 | 33379.6 |
| o-Xylene | 20814.7 | 9726.1 |
⚫︎Measurement result of sample 2
The quantitative value of 1,4-Dioxane was calculated by the standard addition method. The ratio of peak area value of 1,4-Dioxane and d8-Dioxane by both carrier gases are shown in Table 4. The EIC peaks and calibration curves of 1,4-Dioxane obtained from cosmetic ingredient by both carrier gases are shown in Fig. 3. The EIC peaks of m/z 88 of 1,4-Dioxane was observed with a good peak shape from the cosmetic ingredient, and the calibration curve showed a linearity of more than 0.99. The quantitative value of 1,4-Dioxane was 1.13 ppm by He carrier gas and 1.11 ppm by N2 carrier gas. These values were close to the indicated concentration.
Table 4 The ratio of peak area value of 1,4-Dioxane to peak area value of d8-Dioxane
| Added concentration of 1,4-Dioxane |
Ratio of peak area value by He |
Ratio of peak area value by N2 |
|---|---|---|
| 0 ppm | 0.645 | 0.620 |
| 250 ppm | 1.268 | 1.259 |
| 500 ppm | 1.822 | 1.786 |
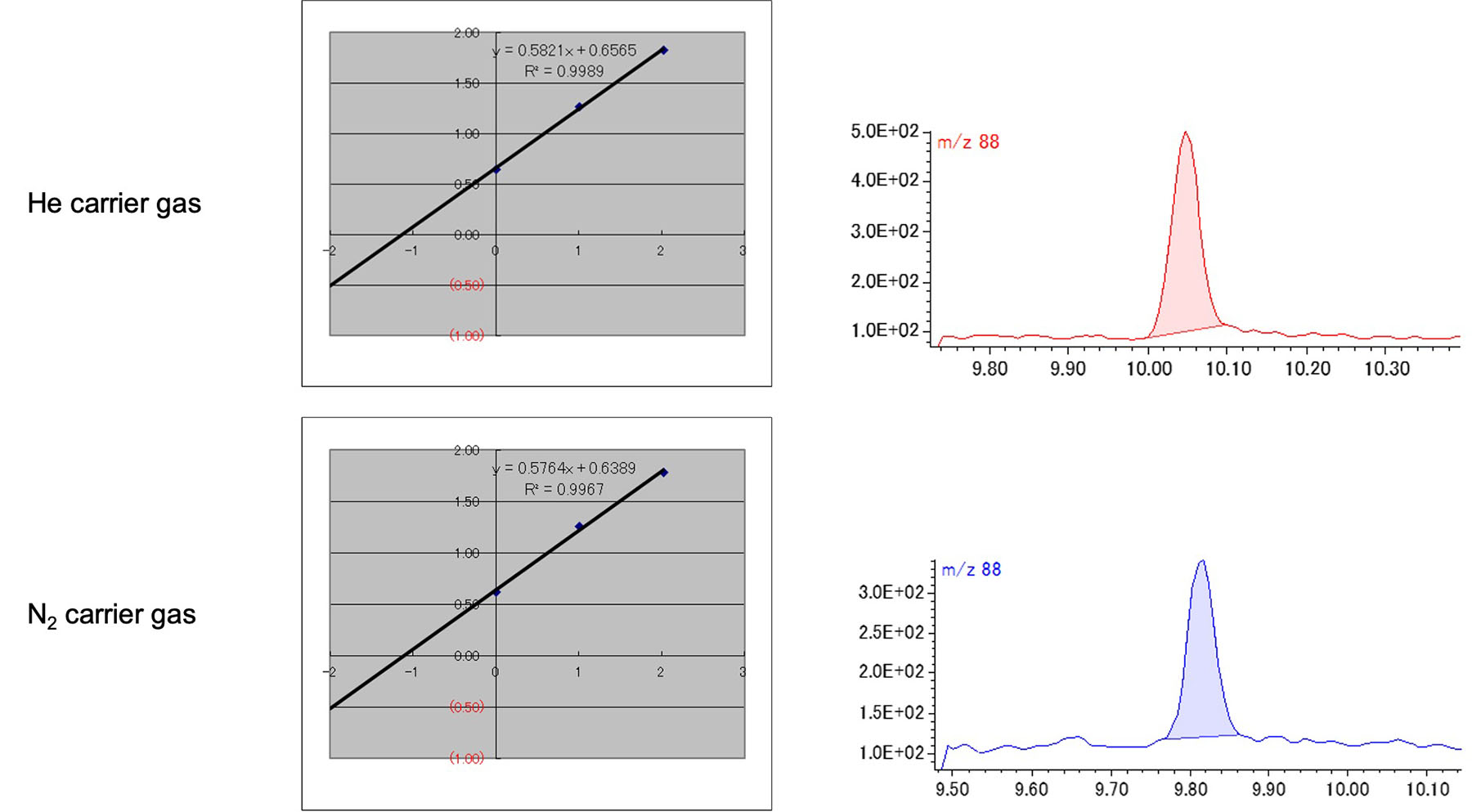
Fig. 3 Calibration curve by standard addition method and the peak of 1,4-Dioxane in cosmetic ingredient
Conclusion
It was shown that residual solvent analysis is possible using the single QMS mode of the JMS-TQ4000GC. Regarding the SIM measurement by N2 carrier gas, it was confirmed that the peak intensity was sufficient for residual solvent analysis. Furthermore, the JMS-TQ4000GC can perform single QMS mode as well as MS/MS mode with a single instrument. Thus, it is possible to select the measurement mode according to the amount of matrix in the sample, making it applicable to a wider range of application fields.
Acknowledgments
We would like to thank Ms. Yasuda, Ms. Kobayashi and Ms. Yamaguchi of KOSÉ Corporation for providing samples for the preparation of this application note.
References
[1] Japanese Pharmacopoeia 18th Edition (2.46 Residual Solvents)
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

