Analysis of calcium oxalate and polystyrene in oxidizing atmosphere using high-resolution TG-TOFMS
MSTips No. 350
MSTips No. 350
Introduction
Thermogravimetric-mass spectrometry (TG-MS) is a measurement technique for obtaining weight change and qualitative information of evolved gas during heating. It is used to evaluate resins and synthetic rubber products. Usually, the inert gas such as helium and nitrogen are used as the TG atmosphere gas. On the other hand, oxidizing atmosphere measurement such as the air will be effective for product development and quality control. However, the electron ionization (EI) method, which is a standard ionization method, is difficult to analyze for a long time under oxygen due to deterioration of the filament. We have developed an oxygen-resistant filament to solve this issue. We have introduced the oxygen-resistant filament application note for QMS in MSTips No. 320. In this MSTips, we introduce anti-oxygen filament application data for high-resolution TOFMS.
Measurement
STA2500 Regulus (NETZSCH) was used as TG. It can also be used for differential thermal analysis (DTA). JMS-T2000GC equipped with an oxygen-resistant filament was used as the MS (Figure 1). The calcium oxalate (①) and Polystyrene (②) were used as test samples. Helium or oxidizing gas (helium : oxygen = 4 : 1 mixed) was used as the TG atmosphere gas, and we have compared these results from two types of the atmosphere gases. Table 1 shows the TG-MS measurement conditions.

Figure 1. JMS-T2000GC with TG
Table 1. Measurement conditions
| Sample | ①Calcium oxalate 20mg |
|---|---|
| ②Polystyrene 5mg | |
| TG | STA 2500 Regulus (NETZSCH) |
| Furnace temp. | ①60°C → 20°C/min → 1,000°C |
| ②60°C → 20°C/min → 600°C | |
| Transfer-line temp. | 300°C |
| Transfer-line column | Blank capillary tube, 3m, I.D.0.32mm |
| Atmosphere | Pure He, 100mL/min |
| Oxidizing (He : O2 = 4 : 1), 100mL/min | |
| Split ratio | 30 : 1 |
| MS | JMS-T2000GC (JEOL) |
| Ionization | EI, Ionization Energy 70eV, 100μA |
| Mass range | m/z 10~800 |
| Ion source temp. | 300°C |
| GC-ITF temp. | 300°C |
Results - ① calcium oxalate
Figure 2 shows the TG curves of calcium oxalate. The black line in each graph is showing pure helium data, and the red line is showing oxidizing gas (helium : oxygen = 4 : 1) data. The reaction formula for each weight loss in a pure helium is as follows.
[1]CaC2O4H2O → CaC2O4+H2O
[2]CaC2O4 → CaCO3+CO
[3]CaCO3 → CaO+CO2
In oxidizing gas, reaction of [2] also produces CO2.
CO+1/2O2→CO2
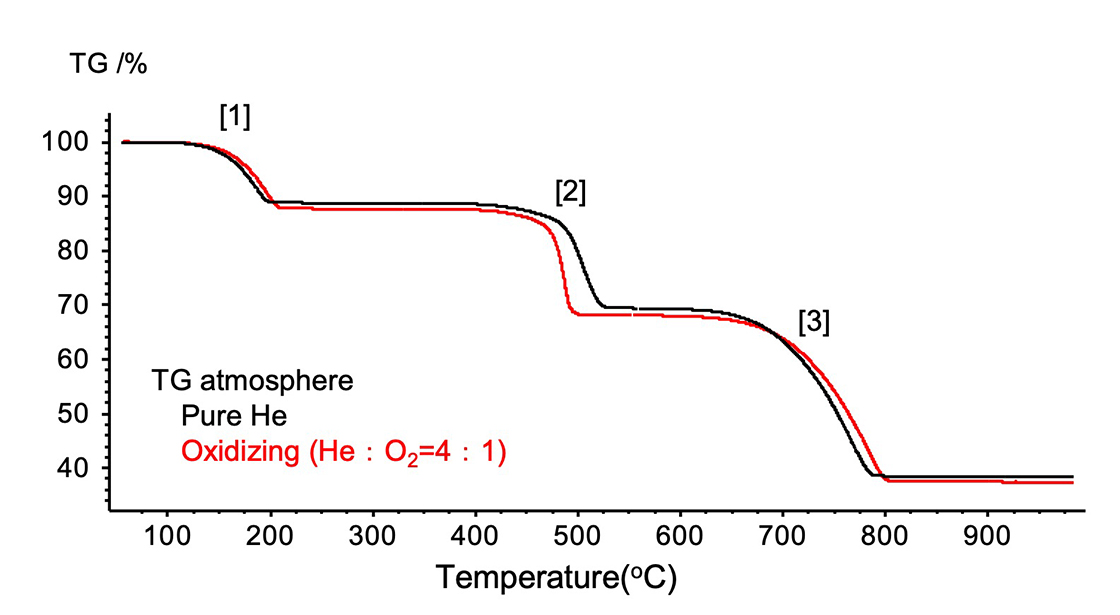
Figure 2. TG curves of Calcium oxalate
Figure 3 shows the DTA curves. It was confirmed that the reaction of [2] became exothermic in oxidizing gas.
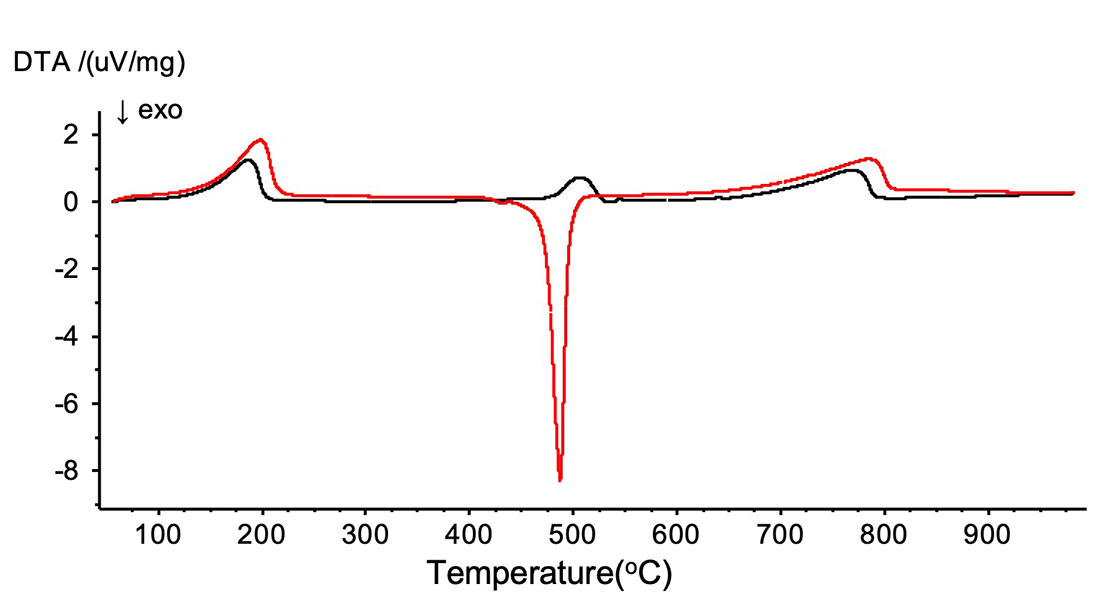
Figure 3. DTA curves of Calcium oxalate
Figure 4 shows the TIC chromatograms. A change in peak shape was observed.
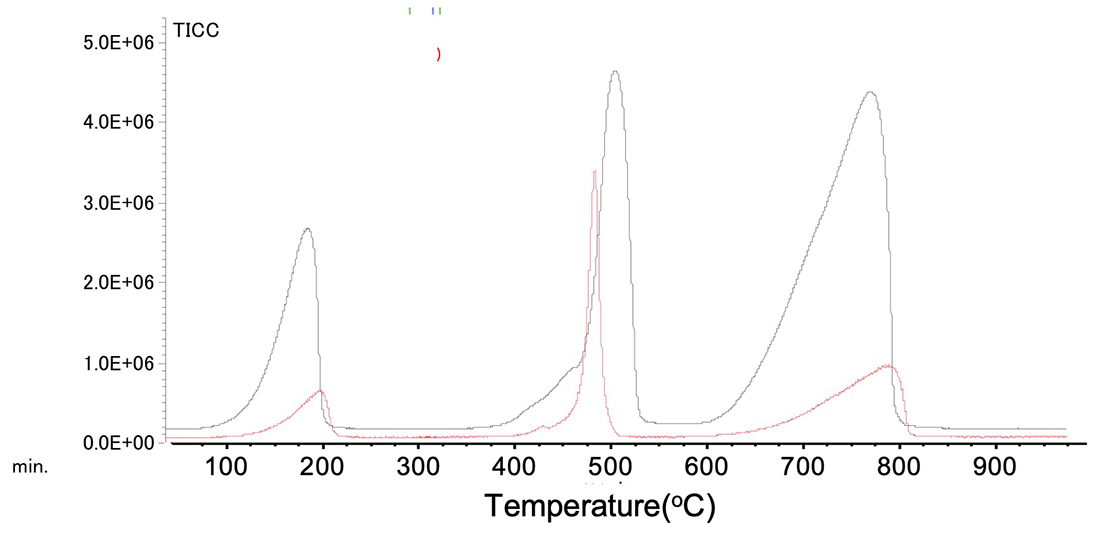
Figure 4. TIC chromatograms of Calcium oxalate
Figure 5 shows the mass spectra of peaks [1] to [3] of calcium oxalate. In the oxidizing atmosphere, the CO signal decreased and the CO2 signal increased due to the reaction [2]CO+1/2O2→CO2.
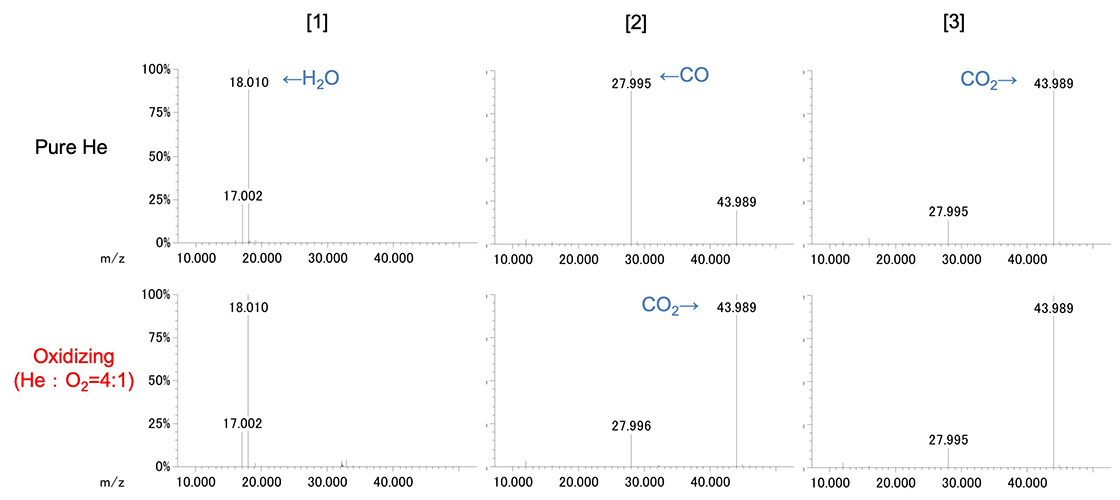
Figure 5. Mass spectra of Calcium oxalate
Results - ② polystyrene
Figure 6 shows the TG curve of polystyrene and Figure 7 shows the TIC chromatogram. The starting temperature of the reaction was lower in the oxidizing atmosphere.
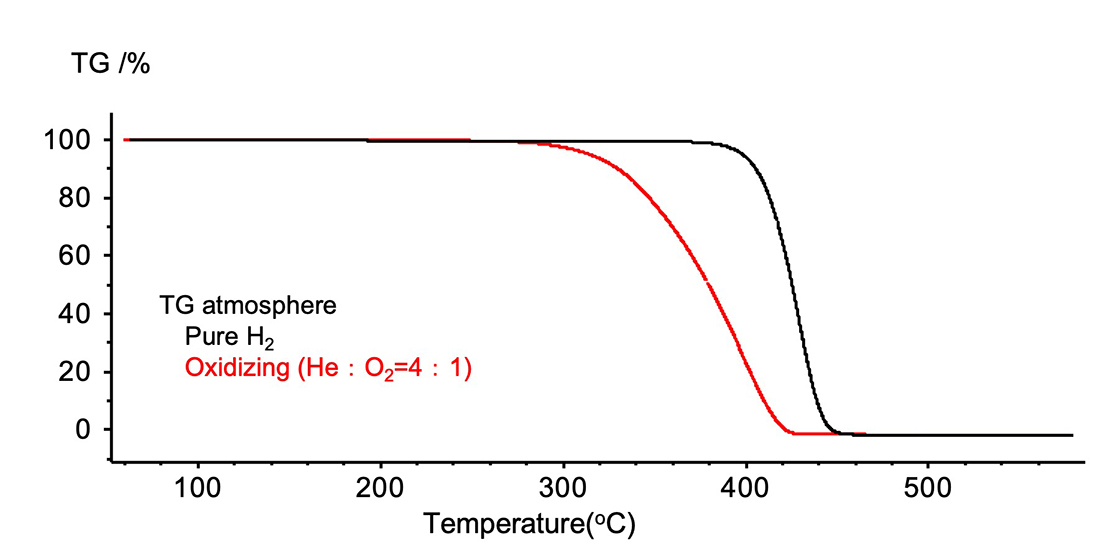
Figure 6. TG curve
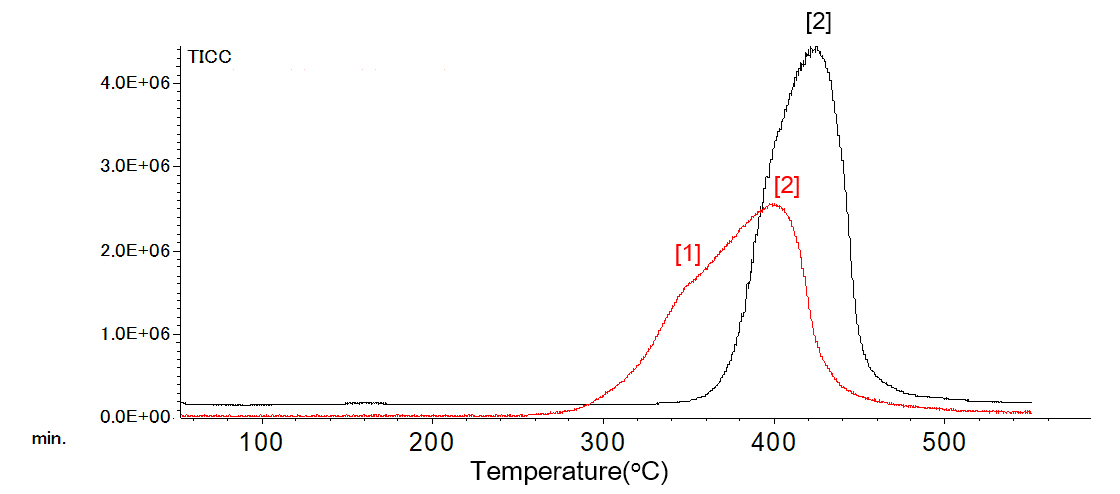
Figure 7. TIC chromatograms of Polystyrene
Figure 8 shows the mass spectra of polystyrene peaks [1] and [2]. In the pure helium atmosphere, major pyrolysis products of polystyrene such as toluene, styrene and styrene dimer were observed. In the front peak [1] of oxidizing atmosphere, the peaks of styrene oxide (= benzaldehyde) and styrene dimer oxide were observed. In the rear peak [2], toluene, styrene, styrene dimer were observed as in the pure helium atmosphere.
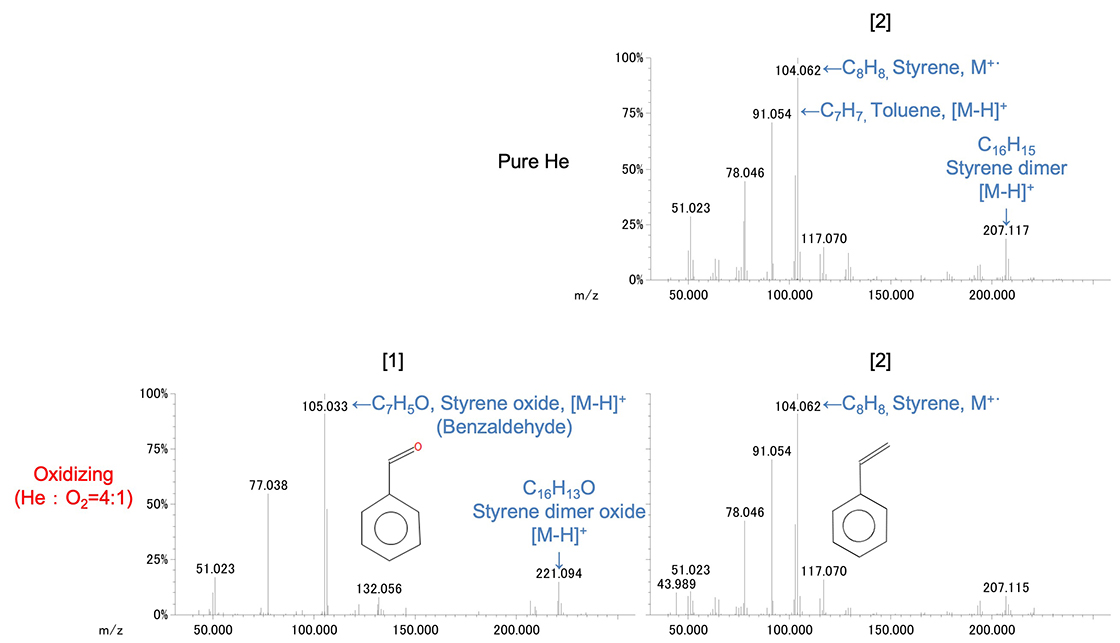
Figure 8. Mass spectra of Polystyrene
Figure 9 shows the TIC chromatogram and the extracted ion chromatogram (EIC) for the base ions of benzaldehyde (m/z 105.03) and styrene (m/z 104.06) in the oxidizing atmosphere. Benzaldehyde was produced in the front (low temperature) and styrene in the rear (high temperature).
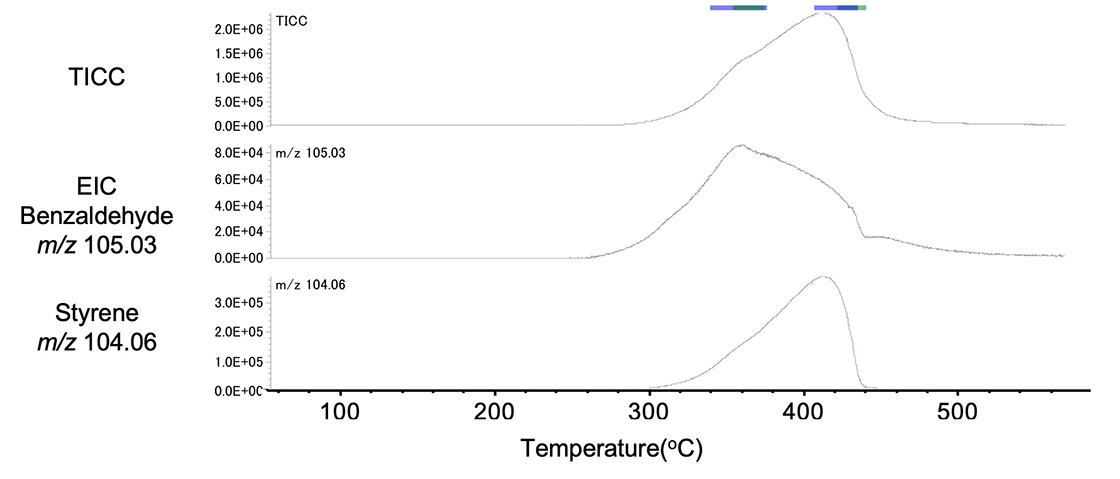
Figure 9. TIC chromatogram and EIC of Polystyrene in oxidizing atmosphere
Conclusion
Measurements were performed in an oxidizing atmosphere with a JMS-T2000GC equipped with an oxygen-resistant filament. As a result, the oxidation reaction of calcium oxalate and polystyrene were observed. In these measurements, the life-time of the filament in an oxidizing atmosphere was more than 50 hours, and it was confirmed that there was no problem with durability. Analysis under an oxidizing atmosphere using this TG-MS system is effective for analysis of various resins and synthetic rubbers, and can be used in product development and quality control.
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

