Evolved gas analysis from polystyrene in an oxygen atmosphere using a filament for low vacuum
MSTips No.320
MSTips No.320
Overview

Figure.1 Configuration of TG/DTA-MS system
The thermogravimetry (TG)/differential thermal analysis (DTA)-mass spectrometry (MS) system can measure the weight change and heat input/output during sample heating with TG/DTA, and simultaneously measure the evolved gas with MS. In TG/DTA, it is possible to use not only inert gases such as helium and nitrogen, but also oxygen-containing auxiliary gases such as air to observe the thermal behavior of different atmospheric gases.
Electron ionization (EI), which is widely used in MS, is an ionization method that utilizes thermal electrons from a filament. In an atmosphere with a large amount of oxygen, such as air, it is difficult to perform stable measurements over a long time because the filament wire tends to break due to thermal oxidation reactions.
JEOL has developed a filament for low-vacuum that utilizes a new wire with enhanced oxidation resistance to enable stable measurement by the EI method in an oxygen atmosphere.
In this study, the gases evolved from polystyrene at various oxygen concentrations were measured by TG/DTA-MS using a filament for low-vacuum.
Instruments
A TG/DTA-MS system (Figure 1) was used for the measurements, which consisted of a NETZSCH TG/DTA STA2500 connected to a JEOL JMS-Q1500GC gas chromatograph quadrupole mass spectrometer. The STA2500 and JMS-Q1500GC are connected by a capillary tube covered by a temperature-controlled transfer line.
The JMS-Q1500GC can be equipped with two filaments in the ion source, and the filament used for the measurement can be switched by the method setting. In this measurement, one filament was replaced with a low-vacuum filament (P/N: 782305971). The filament for low vacuum can be used with the JMS-TQ4000GC gas chromatograph triple quadrupole mass spectrometer as well as the JMS-Q1500GC/JMS-Q1600GC.
Experiments
Small pieces of polystyrene weighed to about 1±0.2 mg in an aluminum pan were used for the measurements. Table 1 shows the measurement parameters for TG/DTA and MS. For the ambient gas for TG/DTA, pseudo-air (helium/oxygen = 4/1) and He were connected to the two purge gas lines, and the oxygen concentration was set to 0, 2, 5, 8, and 10% (v/v) by changing the flow rates of both gases. Furthermore, by connecting pseudo-air to all gas lines, oxygen concentrations of 20% (v/v) can be set.
Table.1 Measurement conditions of TG/DTA-MS system
| TG | |
|---|---|
| Furnace temp. | 100°C → 20°C / min → 600°C |
| Transfer line temp. | Interface 300°C, Line 300°C |
| Purge gas flow | 100 mL / min, Oxygen conc. 0~20% (v/v) |
| GC | |
| Oven temp. | 300°C |
| Column | Blank tube, 2.5 m × 0.25 mm i.d. |
| Column gas flow | 2 mL / min |
| MS | |
|---|---|
| Ion source temp. | 250°C |
| Interface temp. | 300°C |
| Ionization mode | EI, 70 eV |
| Ionization current | 30 μA |
| Relative EM voltage | +200 V |
| Measurement mode | Scan |
| Scan range | m/z 35~500 |
Results
TIC chromatograms measured at each oxygen concentration are shown in Figure 2. The peak of evolved gas due to the decomposition of polystyrene was observed in each TIC chromatogram. The temperature of evolved gas decreases with increasing oxygen concentration, indicating that the pyrolysis temperature of polystyrene varies with oxygen concentration.
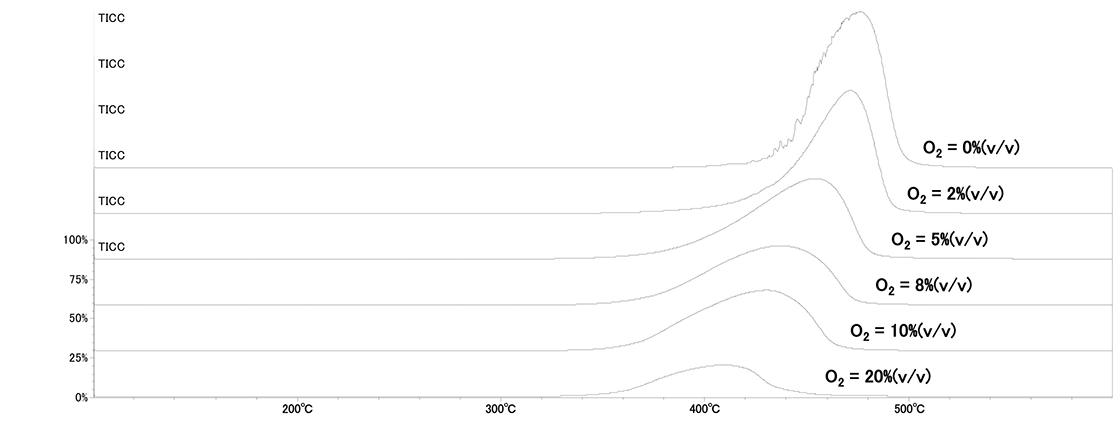
Figure.2 TICC at each oxygen concentration
Figure 3 and Figure 4 show the EICs of the masses corresponding to molecular ions for styrene monomer (M.W.=104) and benzaldehyde (M.W.=106) among the pyrolysis products of polystyrene. The peak area of benzaldehyde increases with increasing oxygen concentration, while that of styrene monomer decreases. From this, it can be assumed that benzaldehyde is formed by the oxidation of styrene monomer.
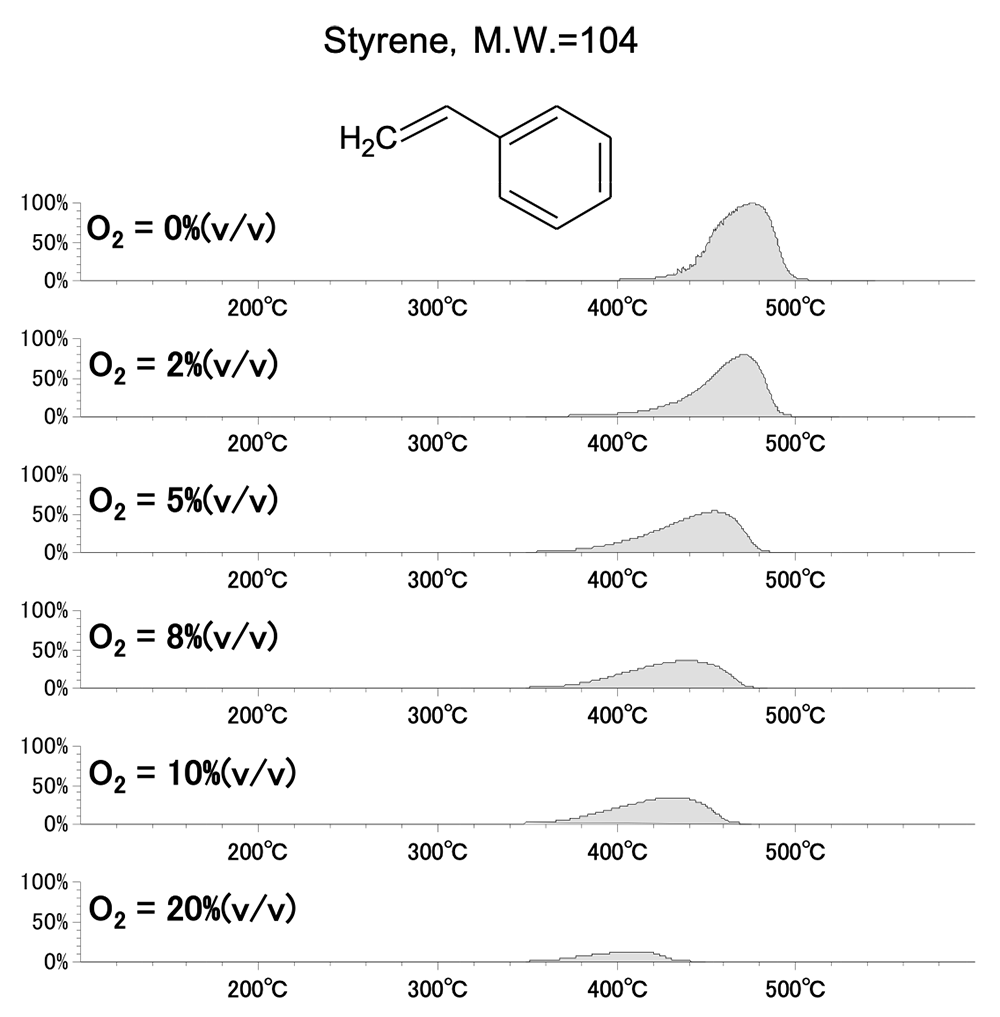
Figure.3 EIC (m/z 104) at each oxygen concentration
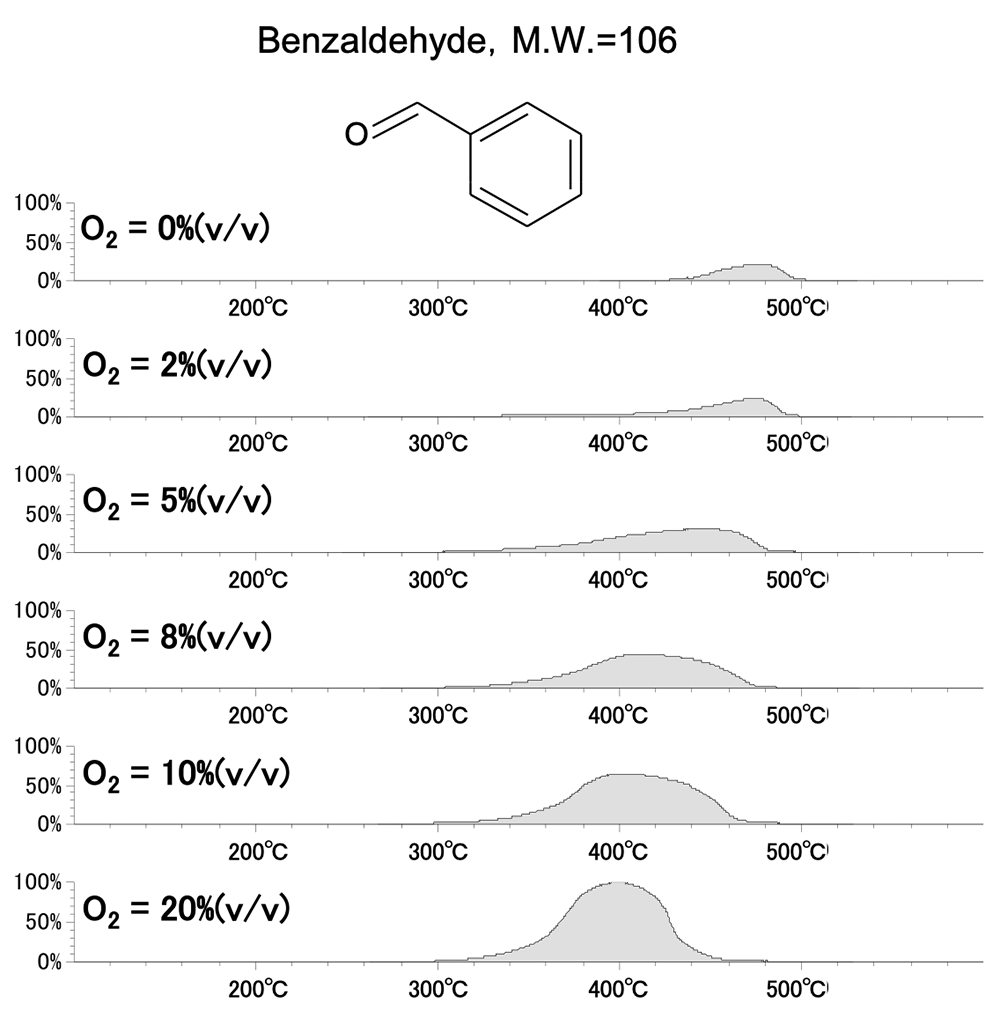
Figure.4 EIC (m/z 106) at each oxygen concentration
Summary
Polystyrene was analyzed under various oxygen concentrations using a TG/DTA-MS equipped with a filament for low vacuum which has a high oxidation resistance. As a result, it was observed that the pyrolysis temperature and the amount of pyrolysis products were dependent on the oxygen concentration.
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

