chemical shift
chemical shift
Chemical shift means the change of the onset energy (threshold energy) of a core-loss spectrum obtained by Electron Energy-Loss Spectroscopy (EELS), which depends on the bonding states and the valence number of an excited atom (chemical environment).
The threshold energy is determined by the energy difference between the core level (initial state) and the lowest energy level in the unoccupied band (the final state). The energies of the initial and final states change with the chemical environment.
As an example, consider the case of the core-electron excitation spectrum of a metal atom in a metal and its oxide (Fig. 1). In the case of the metal, the spectral threshold energy is given by the energy difference between the core level, from which an electron is excited, and the level just above the Fermi level. In the case of a metal-oxide, the valence electron density on the metal atom decreases because electrons move from the metal atom to the oxygen due to the metal-oxygen bonding. As a result, the electrostatic potential energy of the inner shell electrons due to the nucleus increases (the screening of the nuclear potential with the valence electrons decreases), leading to a shift of the core level (initial state) to the low energy side. Thus, the energy difference between the initial state and the final state becomes large, and the threshold energy shifts to the high energy side. Among the different oxides of the same metal, as the valence number of the metal ion is higher, the shift of the core level to the low energy side tends to be larger. In addition, for the insulating oxide, as an energy gap appears near the Fermi level, the bottom energy of the unoccupied band (final state) shifts to the high energy side. Therefore, the threshold energy of the oxide becomes larger than that of the metal, giving a positive chemical shift (shift to the high energy side). Fig. 2 shows examples of chemical shift appearing in the EELS spectra of manganese obtained from various manganese oxides. The spectra elucidate that, as the valence number of manganese is larger, the spectral peak is seen to shift by a few eV to the high energy side.
The above description assumes that the transition of the electron occurs from the ground state where the core electron is not excited. However, in real experiments, the influence of the core hole, which is created by the excitation of the core electron, should be taken into account. The core hole gives rise to an attraction-type Coulomb potential and the electrons near the core hole are attracted to the core hole to screen the Coulomb potential. This can lead to the shift of the core level to the high energy side, but this effect is regarded to be small.
For the insulating material, it is known that the core hole and the excited electrons often form a bound state (core exciton). The exciton level that appears in the band gap may become the final state for EELS. In such a case, the threshold energy decreases by the energy value of the binding energy of the exciton. The binding energy of the core exciton depends on materials, but ranging from about 100 meV to beyond 1 eV. When the binding energy of the core exciton is large, a spectral peak is observed as a separate peak just below the absorption edge. Since the chemical shift measured by EELS is affected also by the change in the final state, the interpretation of the EELS spectra is not easy. If the spectra of standard materials can be obtained in advance, it is possible to use the chemical shift to determine the valence number.
It should be noted that X-ray Photoelectron Spectroscopy (XPS) provides also information on chemical shift arising at ionization of a core electron. The final state of XPS exists in vacuum outside the specimen. Thus, the final state always stays the same and does not contribute to the chemical shift in XPS, this situation being different from the case of the core-hole interaction in EELS. Therefore, the spectral interpretation of chemical shift by XPS is easier than that by EELS.
(By Professor Hiroki Kurata, Kyoto University)
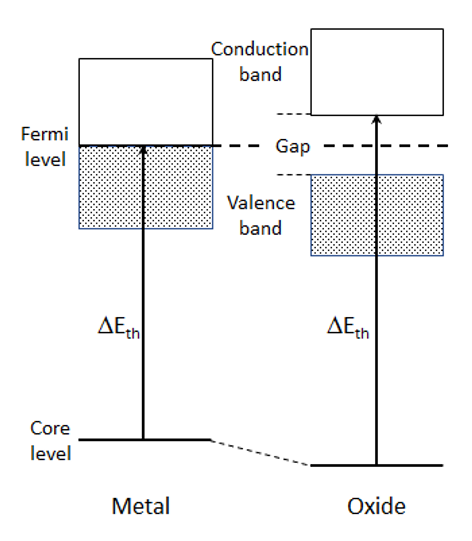
Fig. 1
Threshold energies of a core-loss spectrum obtained from metal at the ground state and from its oxide.
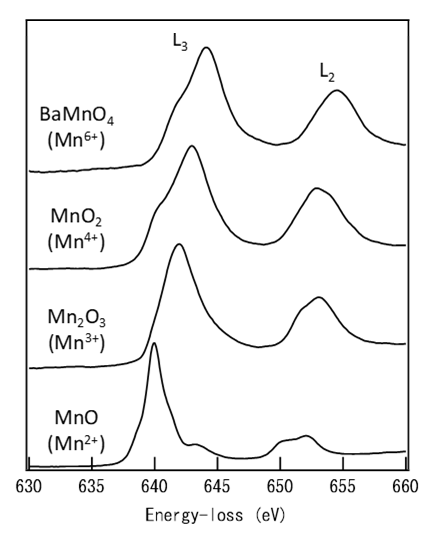
Fig. 2
L2,3-shell excitation spectra of various manganese oxides. As the valence number of manganese is higher, an L2,3-absorption edge shifts to the high energy side.
Related Term(s)
Term(s) with "chemical shift" in the description
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.