Assessing UV Degradation of Polymers: A Study of Polyethylene Terephthalate by using MALDI-TOFMS and GC-TOFMS
Introduction
Polyethylene terephthalate (PET) has extensive applications across various industries due to its versatility and desirable properties. It is commonly used in food packaging, beverage containers, fibers, and more. PET's transparent, lightweight, and durable nature makes it an ideal material for products such as beverage bottles and food containers, ensuring product safety and preservation.
Additionally, PET fibers are utilized in clothing and household items, providing durability and flexibility.
Conducting degradation analysis on PET products is important in ensuring quality control and maintaining long-term performance. PET is susceptible to degradation due to environmental factors, which can alter its physical and chemical properties over time. Through degradation analysis, the durability and safety of products can be evaluated, facilitating improvements in manufacturing processes and materials. Furthermore, degradation analysis is crucial in assessing environmental impact and promoting recyclability, contributing to sustainable product development efforts.
Mass spectrometry is a powerful tool for polymer characterization and can be applied to polymer degradation analysis. In this work, PET films that were degraded by ultraviolet light were evaluated by high-resolution matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOFMS) and gas chromatography (GC)-TOFMS.
Preparation of UV Degraded PET Film
Figure 1. Deterioration of PET film by UV lamp.
A commercially available PET film was used as the sample.
The PET film was irradiated with UV from one side using a Portable Cure 100, illuminance: 170 mW/cm2, (Sen Light Corporation).
MALDI-TOFMS Measurement

JMS-S3000 SpiralTOF™-plus3.0
MALDI-TOFMS is a powerful tool for the analysis of polymers. Since MALDI most commonly produces single charged ions, the m/z in the mass spectrum represents the mass of a polymer ion. High mass resolution MALDI-TOFMS can be used to identify polymer series with different repeating units, end group compositions, and molecular weight distributions in a given sample. JEOL's MALDI-TOFMS, JMS-S3000 “SpiralTOF™-plus 3.0” is an ultra-high resolution TOFMS with a unique spiral ion optical system. It is ideal for polymer analysis because it can achieve high mass resolution over a wide mass range. In addition, when analyzing PET films, it is possible to identify degradation areas by using mass spectrometry imaging (MSI) technology. First, the UV-degraded PETs were measured by dissolving the samples in an hexafluoroisopropanol solution.
The mass spectra for the 0-, 8-, and 54-hours UV irradiation times are shown in Figure 2a-c. The main polymer series observed in 0- and 8-hour UV irradiation was cyclic oligomers (★). However, for the 54-hour UV irradiated sample, polymer series with COOH/COOH end-groups (Figure 2c) (★★★) were observed that were the result of photooxidation.
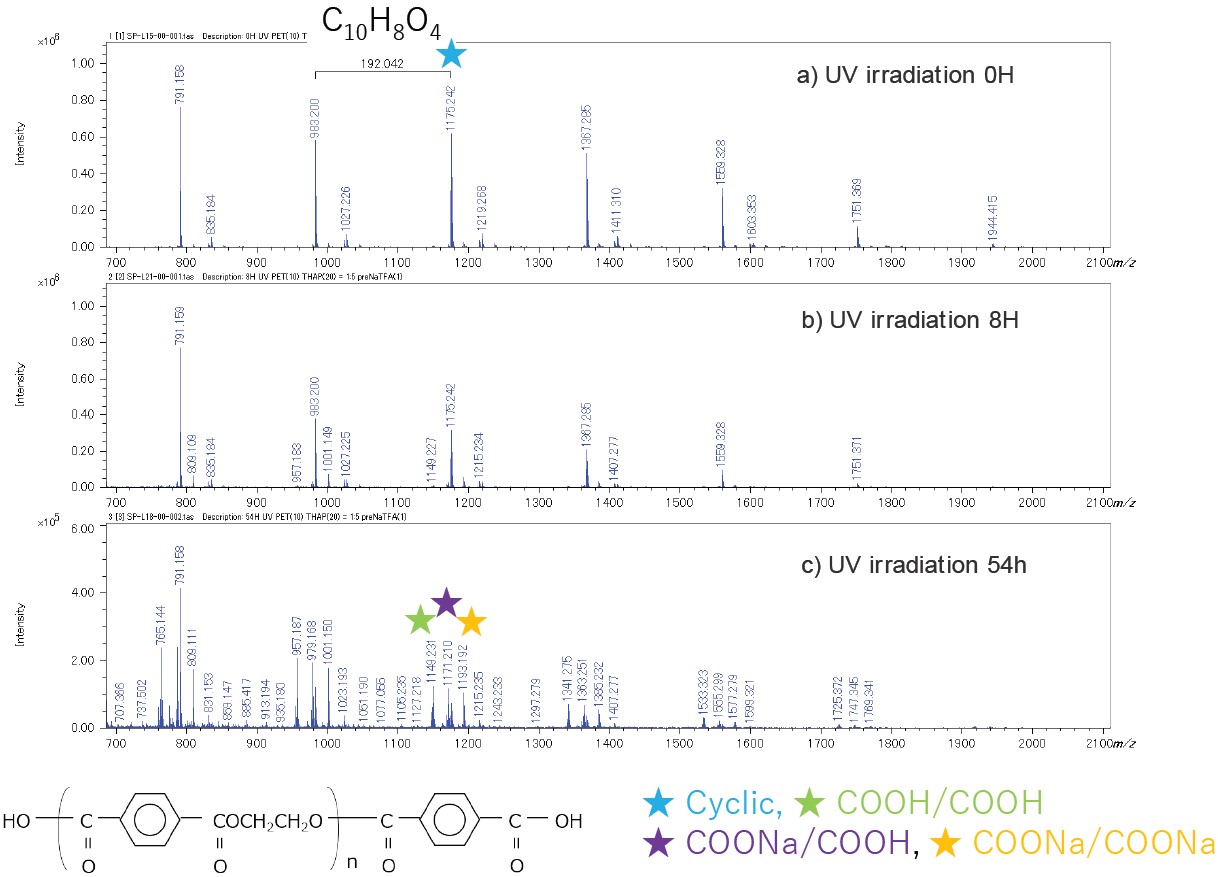
Figure 2. Mass spectra variation due to UV degradation of whole PET films.
The cyclic oligomers were decreased and oligomers with COOH/COOH end-groups were appeared after 8 hour.
Next, the PET film surface was prepared for analysis by directly adding matrix solution dropwise to the surface and allowing it to dry. The mass spectra measured from the surface are shown in Figure 3. In this case, the COOH/COOH end-groups appeared after only 0.5 hours of UV exposure, which means photooxidation had already started occurring at the surface of the film. Next, the PET film surface was analyzed by using MSI. For these experiments the left half of the PET film was exposed to UV irradiation for 0.5 hours, and the right half was covered to prevent UV degradation of the surface. Afterwards, MSI measurements were performed to show the extracted m/z images for the cyclic oligomers (Series I), COOH/COOH (Series II), and normalized COOH/COOH to cyclic oligomers.
These results show that MSI can effectively monitor the UV degradation of PET film surfaces.
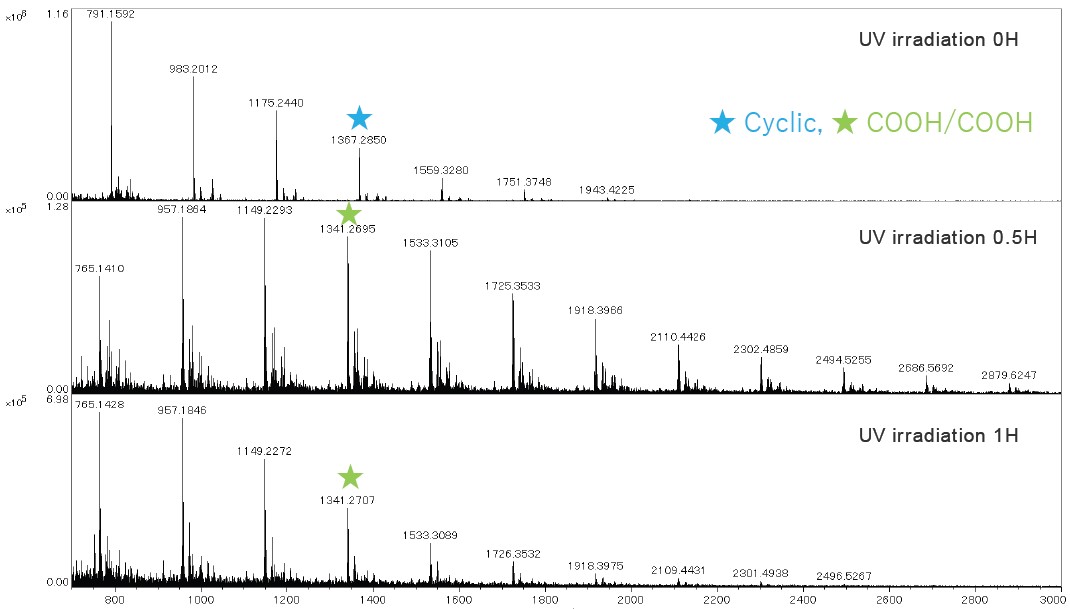
Figure 3. Mass spectra variation due to UV degradation of PET film surface. The cyclic oligomers were
decreased and oligomers with COOH/COOH end-groups were appeared at 0.5 hour earlier than whole PET film analysis.
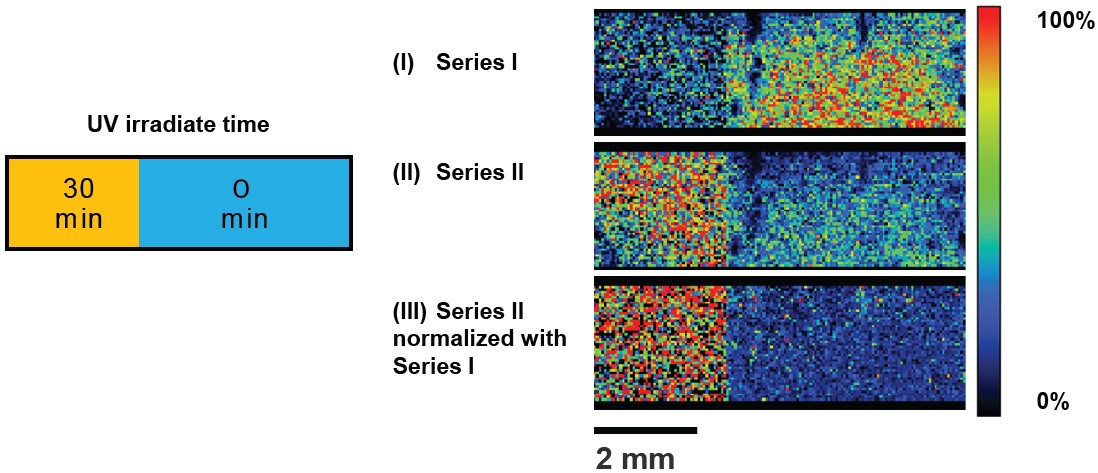
Figure 4. Mass spectrometry imaging of UV degraded PET films. The degradation parts was clearly identified.
GC-TOFMS Measurement

JMS-T2000GC AccuTOF™-GC Alpha
Pyrolysis GC-MS (Py-GC-MS) is a method in which polymeric materials are instantaneously heated in an inert atmosphere, and then the pyrolysis products are analyzed by GC-MS. When the target is a condensation polymer containing ester bonds, multiple decomposition peaks (mainly polar compounds) are observed due to thermal decomposition reactions, thus making it challenging to perform a highly accurate analysis that reflects the polymer structure itself. To handle this, a reactive Py-GC-MS method can be used to effectively minimize these problems for condensation polymers. In this method, a reaction reagent such as an acid or alkali is introduced and heated together with the sample to selectively cleave and derivatize the ester bonds, simultaneously, thus simplifying the Py-GC-MS results.
Consequently, the UV-irradiated PET film samples were measured with reactive Py-GC-TOFMS using both electron ionization (EI) and field ionization (FI) with a combination EI/FI
ion source. The structural analysis was performed with msFineAnalysis AI.
Figure 5 shows the total ion current chromatogram (TICC) using EI for the 0-hour (blue line) and 10-hour (red line) UV-irradiated PET films. Three main compounds (A, B, C) were strongly observed that represent the reactive pyrolysis products derived from the main chain of PET.
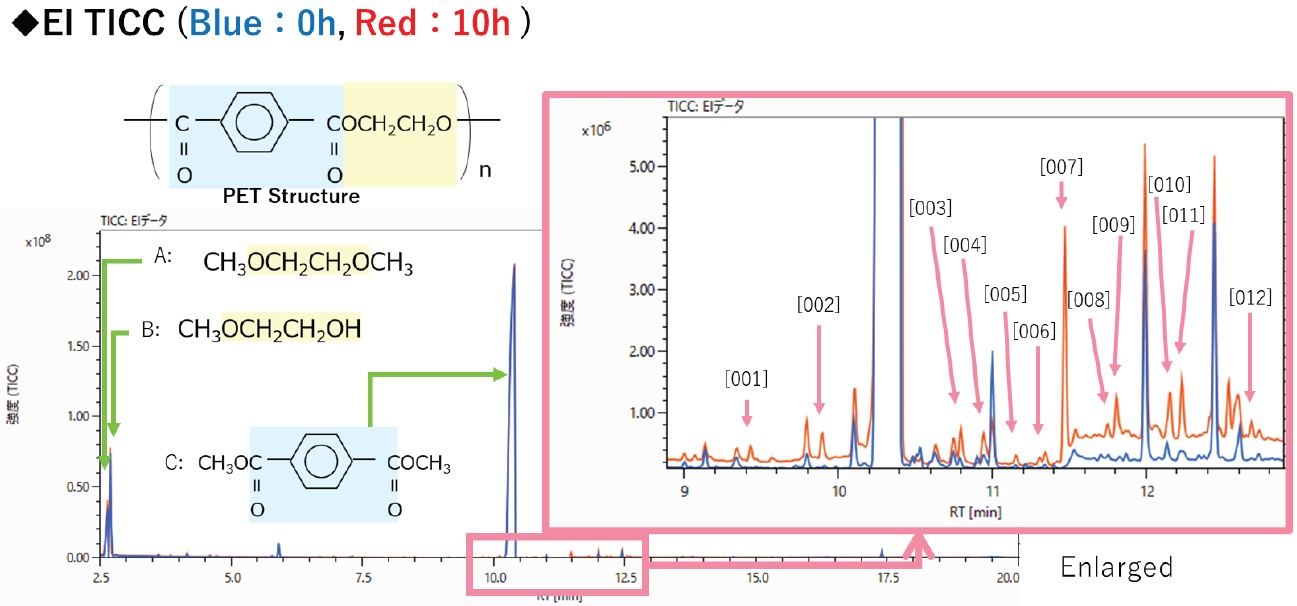
Figure 5. TICC of reactive Py GC-TOFMS of PET films of UV irradiation time 0 and 10 hours.
As a result of performing a difference analysis of the two samples, 12 components were observed at retention times between 9-13 min in the TICC that were characteristic to the UV irradiated sample.
An extracted ion chromatogram (EIC) was created from the base peak of ID: 007, which was a major characteristic compound after UV irradiation. The variation of the intensity for this compound at different UV irradiation time showed that PET degradation progressed linearly with the UV exposure time (Figure 6). It was also found that this method effectively showed the changes due to UV irradiation, even for the earlier exposure times.
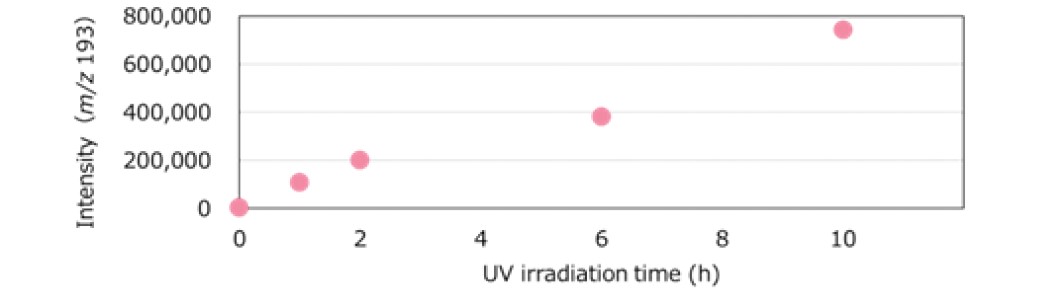
Figure 6. The ion intensity variation of characteristic peaks of PET degradation.
Finally, a structure analysis was performed for each of the 12 characteristic compounds. As a starting point, a NIST library database search was performed for all 12 components, and 3 components (ID:001, 003, 010 in the table below) showed a match factor of 750 or higher to support their identification. For the other 9 components that did not show a good match in the NIST library database, an automatic structure analysis was performed using the AI structure analysis function provided with msFineAnalysis AI (Table 1).
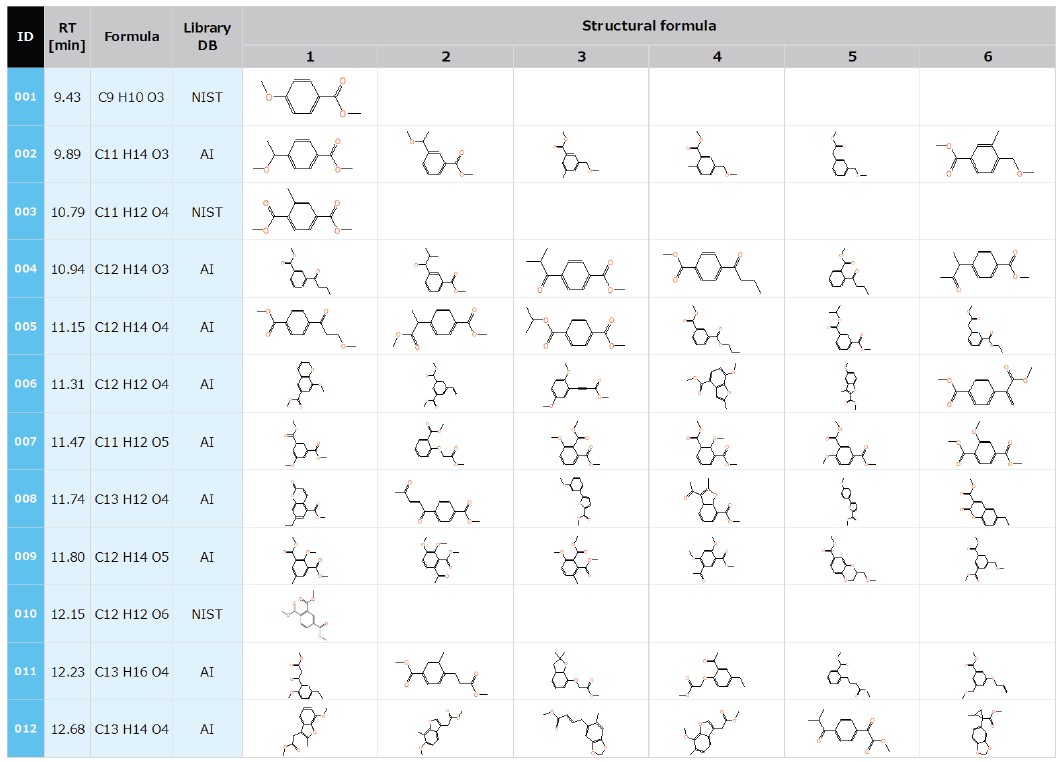
Table 1. The structure estimation of reactive PY GC-TOFMS data with msFineAnalysis AI.
Conclusions
Detailed characterization of UV-degraded PET was performed by combining MALDI-TOFMS and reactive pyrolysis GC-TOFMS results.
MALDI-TOFMS analysis confirmed that the polymer series was derived from UV degradation that included COOH/COOH end groups. This data also showed that it was possible to observe the early stages of UV degradation by using MSI measurements of the sample surface.
Structural analysis of reactive pyrolysis products from UV-degraded PET was performed by integrated qualitative analysis of Py-GC-TOFMS. Only 3 of the 12 reactive pyrolysis products characteristic of UV degradation were registered in the NIST library, so an AI structural analysis was performed for the other 9 analytes to determine possible structures. Additionally, the early-stage degradation was observed by focusing on one of these unique compounds.
Solutions by field
Related products
Product category
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.


