Rapid mass analysis of organic pigments in toner using DEP
MSTips No. 489
Introduction
Toner used in laser printers and multifunction machines (copy machines) is mainly composed of three components: polymer resin, wax, and pigment for coloring. There are four colors of toner for color printing: CMYK (cyan, magenta, yellow, and black), and pigment plays an important role in creating various colors. In order to disperse pigments as solids during printing, their chemical structure has a crystalline structure and strong molecular bonds. As a result, they are less soluble in water or common organic solvents, and are also less volatile. As a result, it is difficult to qualitatively analyze the pigment itself using mass spectrometry coupled with chromatography.
DEP is a technique in which a sample is applied directly to the platinum filament at the tip of the probe and the sample is rapidly heated by passing an electric current through the filament. This makes it possible to rapidly ionize the sample while minimizing thermal decomposition. Therefore, sample pretreatment is almost unnecessary, and it is suitable for the analysis of non-volatile substances or substances that are easily thermally decomposed. In this study, we attempted rapid qualitative analysis of the organic pigments contained in toner using DEP.
Experimental
Three types of toner (cyan/magenta/yellow) for a certain copy machine were used as samples. A GC-QMS (JMS-Q1600GC UltraQuad™ SQ-Zeta, manufactured by JEOL Ltd.) was used for the measurement. No sample pretreatment was performed, and the toner was applied directly to the filament of the DEP probe and inserted into the ion source. The EI method was used for ionization. The measurement conditions for the DEI-MS measurement are shown in Table 1.
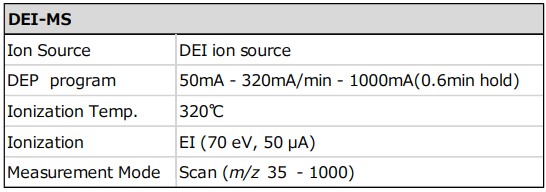
Table 1 Measurement condition
The tip of DEP

JMS-Q1600GC UltraQuad™SQ-Zeta
Result and Discussion
Figure 1 shows the TIC Chromatogram (TICC) and the EIC of m/z 575 obtained by DEI-MS measurement of cyan. Broad peaks with peak tops around at R.T. 0.7, 1.0, 1.3, 1.4, and 1.5 minutes were detected by TICC.
Figure 2 shows the mass spectrum of R.T. 1.32 minutes and the results of the library search using the NIST mass spectrum database. As a result, it was estimated that the organic pigment contained in the cyan toner was copper phthalocyanine.
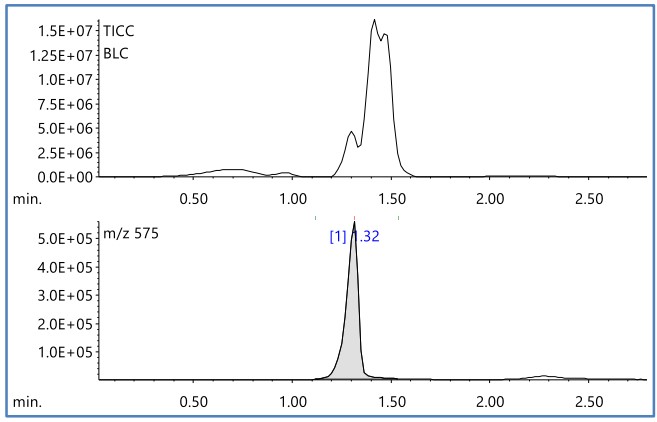
Figure 1 TICC and EIC(m/z 575) of toner(cyan)
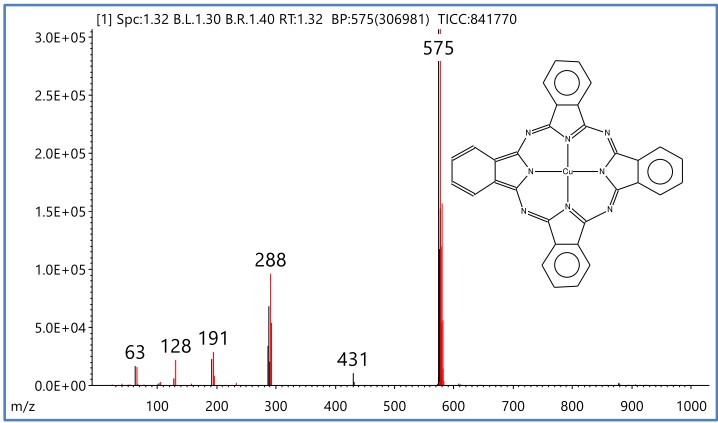
Figure 2 Mass spectrum of R.T.1.32min and NIST library search results
Next, Figure 3 shows the TIC Chromatogram (TICC) and the EIC of m/z 340 obtained by DEI-MS measurement of magenta. Broad peaks with peak tops around at R.T. 0.7, 1.0, 1.3, 1.4, and 1.5 minutes were detected by TICC.
Figure 4 shows the mass spectrum of R.T. 1.27 minutes and the results of library search using the NIST mass spectrum database. As a result, it was estimated that the organic pigment contained in the magenta toner was "2,9-dimethyl-5,6a-dihydroquinolino[2,3-b]acridine-7,14-dione", that is, "Pigment Red 122"
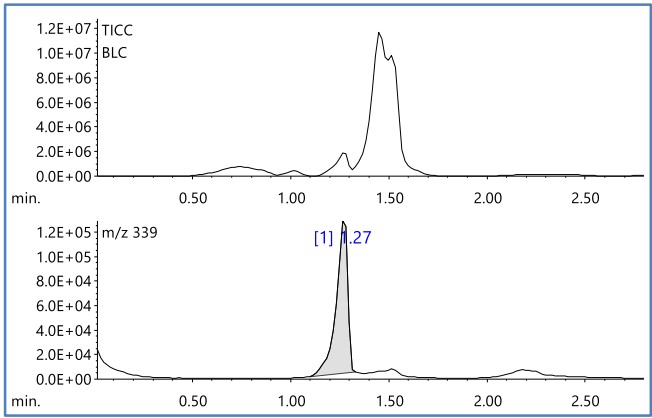
Figure 3 TICC and EIC(m/z 340) of toner(magenta)
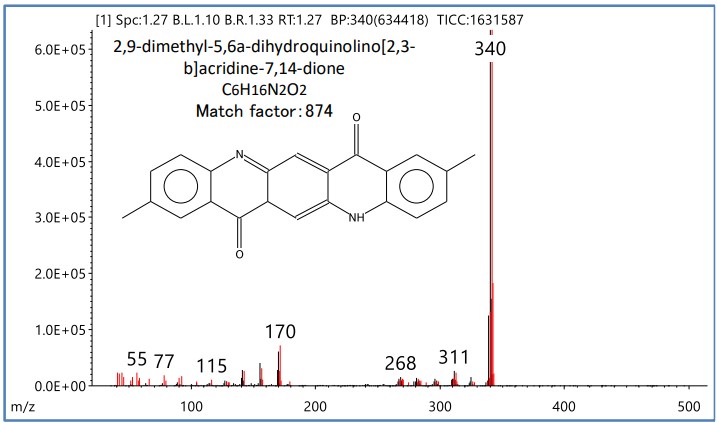
Figure 4 Mass spectrum of R.T.1.27min and NIST library search results
Finally, Figure 5 shows the TIC Chromatogram (TICC) and the EIC of m/z 337 obtained by DEI-MS measurement of yellow. Broad peaks with peak tops around at R.T. 0.8, 1.18, 1.4, and 1.5 minutes were detected by TICC.
Figure 4 shows the mass spectrum of R.T. 1.18 minutes. A library search using the NIST mass spectral database did not yield any significant results. However, if the molecular ion is clearly detected as in the first two types of organic pigments, the molecular ion of this component is also estimated to be 337. Pigment Yellow 185 “2-cyano-N-methyl-2-[3-(2,4,6-trioxo-1,3-diazinan-5-ylidene)-2,3-dihydro-1H-” shown in Figure 7 isoindol-1-ylidene]acetamide” is a strong candidate.
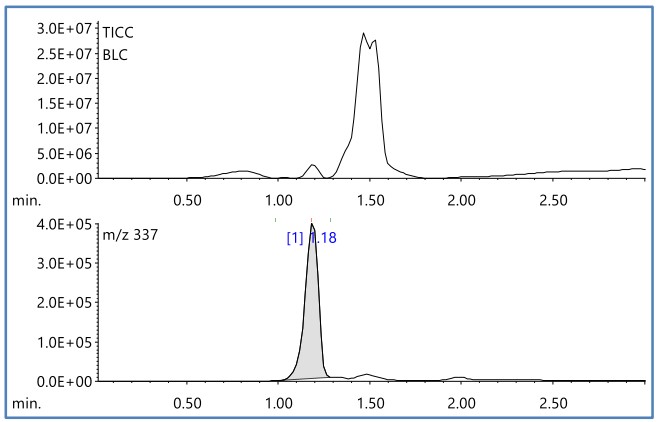
Figure 5 TICC and EIC(m/z 337) of toner(yellow)

Figure 6 Mass spectrum of R.T.1.27min
Furthermore, by analyzing the correlation between the fragment ions in the mass spectrum of Figure 6 and the molecular structure of Pigment Yellow 185, it can be deduced that m/z 307 is the [M-NHCH3]+ ion, and m/z 280 is the [M-(CONHCH3-H)]+ ion. From the above, it was estimated that the organic pigment in this yellow toner was Pigment Yellow 185.
In addition, with regard to the peaks common to all toners, it was inferred from the mass spectrum that the component eluted around R.T. 0.7 minutes was a wax component, which is a sub-resin of the toner, and the peaks at R.T. 1.4 minutes and 1.5 minutes were respectively components related to the polymer resin of the toner.
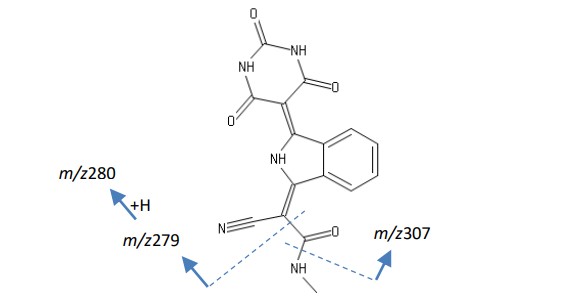
Figure 7 Structural formula of Pigment Yellow 185 (2-cyano-N-methyl-2-[3-(2,4,6-trioxo-1,3-diazinan-5-ylidene)-2,3-dihydro-1H-isoindol-1-ylidene]acetamide)
C16H11N5O4:MW337
Conclusion
In this report, we have shown that mass spectrometry using the DEP method can detect organic pigments that are generally difficult to dissolve in organic solvents. DEP measurements are very useful because they do not require sample pretreatment and can be analyzed quickly, taking only about 3 minutes.
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

