Measurement of boroxine cage using JMS-S3000 “SpiralTOF™”
MSTips No.369
Boroxine cages are nanometer-sized covalent cage-like molecules that utilize boroxine formation reactions [1]. Such molecular-sized hollow structures can contain other molecules. Encapsulated molecules sometimes change their properties significantly, and various applications using them are being investigated. One of the methods for confirming the synthesis of boroxine cages is mass spectrometry, and MALDI-TOFMS is suitable because it can ionize a wide range of compounds mainly as single-charge ions. The JMS-S3000 SpiralTOF™ achieves a long flight distance of 17 m due to its unique spiral ion trajectory, and can measure low-molecular-weight to high-molecular-weight compounds ionized by the MALDI method with high mass resolution and high mass accuracy. In this report, we report the accurate mass measurement of the boroxine caged 12-mer.
Measurement conditions
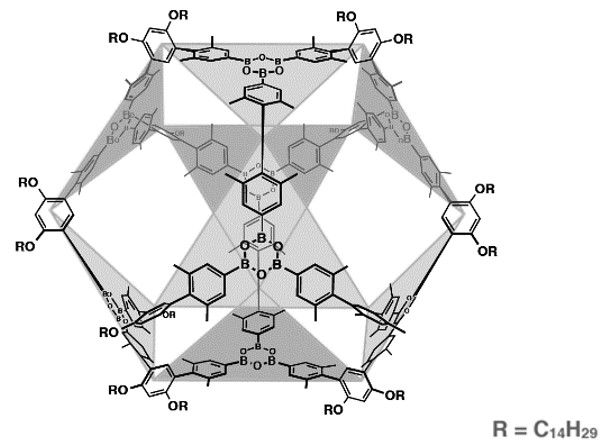
Figure1 Structure of boroxine cage 12-mer.
The sample is a boroxine caged 12-mer (Figure 1). Table 1 shows the elemental composition and mass information. DCTB (trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile) was used as the matrix, and AgTFA (silver trifluoroacetate) was used as the cationizing agent. Mass spectra were acquired in SpiralTOF mode (positive ion mode). For accurate mass measurement, mass calibration was performed using polystyrene as an internal standard.
Table1 Elemental composition and m/z of boroxine cage 12-mer.

Measurement results and summary
Figure 2 shows the mass spectrum in SpiralTOF mode. In the mass spectrum, the boroxine cage 12-mer [M+Ag]+ was observed as the base peak, and the 9-mer [M+Ag]+ was also observed. A close-up view around the isotope peaks of the 12-mer is shown. As can be seen from this, the relative intensity of the monoisotopic peak (m/z 9246) is only 0.0017% in the isotope pattern of the 12-mer with a molecular weight of about 10,000 and cannot be observed. Similarly, the [M+Ag]+ ion of polystyrene used as an internal standard has a low monoisotopic peak intensity, making accurate mass calibration difficult.
Therefore, isotopic peak patterns were simulated for each isotopic cluster of polystyrene used as an internal standard, and the most abundant peak within each isotopic cluster was used to establish a mass calibration. Based on this, the mass error of the most abundant peak of the boroxine-cage 12-mer isotopic cluster was examined, and the error was -0.7 mDa from the calculated mass. It also showed good agreement compared with the simulation results of isotope patterns.
In this way, SpiralTOF™ is an effective means of confirming the synthesis of high-molecular-weight compounds with a molecular weight of ~10,000 Da based on the accurate mass and isotope pattern.
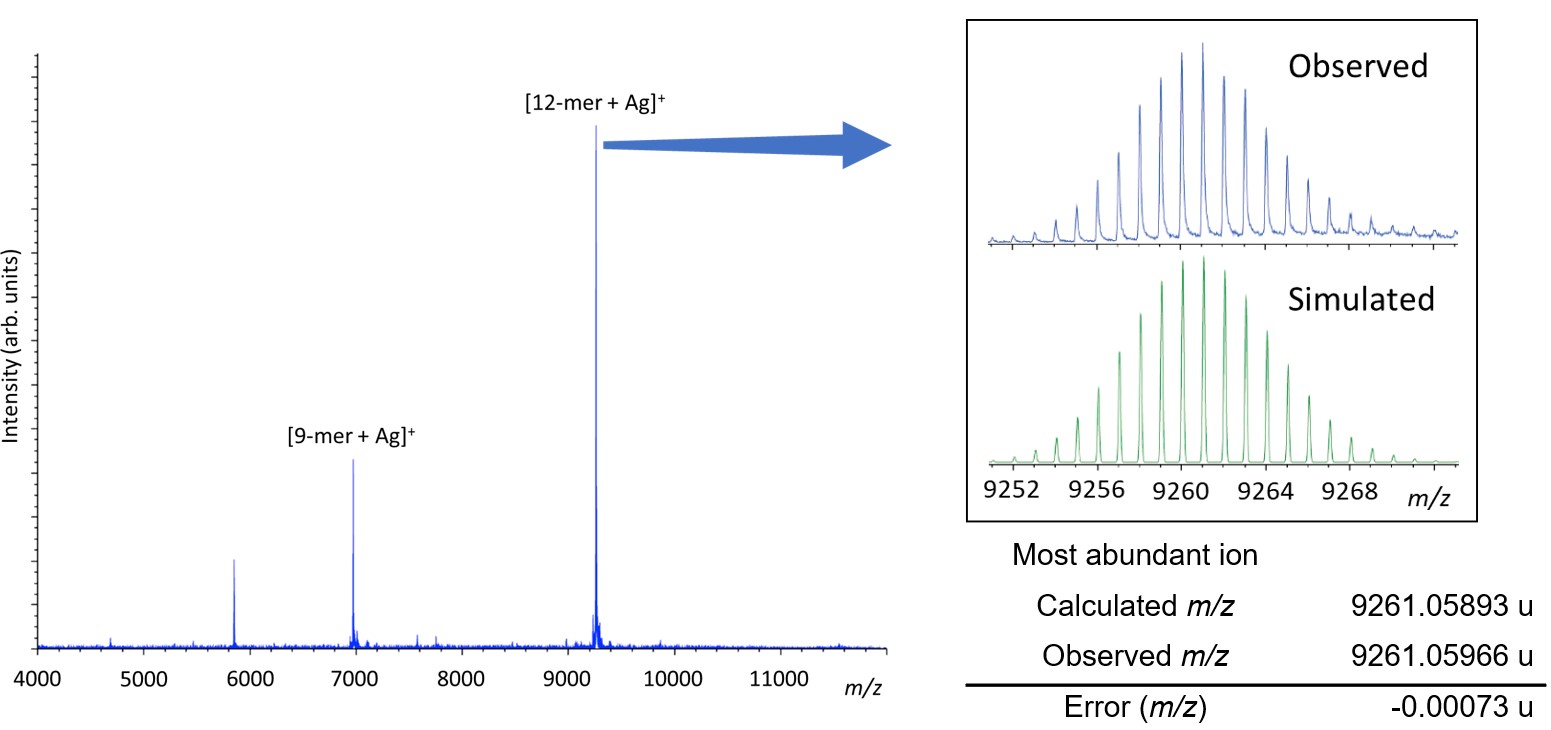
Figure2 Observed and simulated mass spectrum of the boroxine cage 12-mer.
Reference
1) Self-Assembly of Nanometer-Sized Boroxine Cages from Diboronic Acids, K. Ono et. al. J. Am. Chem. Soc. 2015, 137, 7015−7018
Acknowledgement
Sample courtesy of Prof. Iwasawa, The Tokyo Institute of Technology
Solutions by field
Related products
Product category
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

