Analysis of gases generated from all solid-state Li-ion batteries using sulfide-based solid electrolytes
MSTips No. 351
Introduction
Understanding the gases generated inside a rechargeable battery when it is in use is considered in terms of performance maintenance and quality control. By using an experimental battery from which internally generated gases can be extracted, it is possible to identify the gas species generated and estimate changes in the amount of gas generated, although this is different from the actual operating environment of the battery, which is an enclosed space. In this paper, we focus on the gases generated during the charging and discharging of all-solid-state batteries and present an example of analysis using a mass spectrometer.
Method
An experimental cell capable of supplying gas and extracting the generated gas was connected to a mass spectrometer to measure the gases generated after and during charging and discharging. A picture of the connection between the experimental cell and the mass spectrometer is shown below. The gas species were identified using a JMS-T2000GC AccuTOF™ GC-Alpha high-performance gas chromatograph mass spectrometer, and the changes in gas volume were measured using a JMS-Q1600GC UltraQuad™ SQ-Zeta gas chromatograph mass spectrometer.
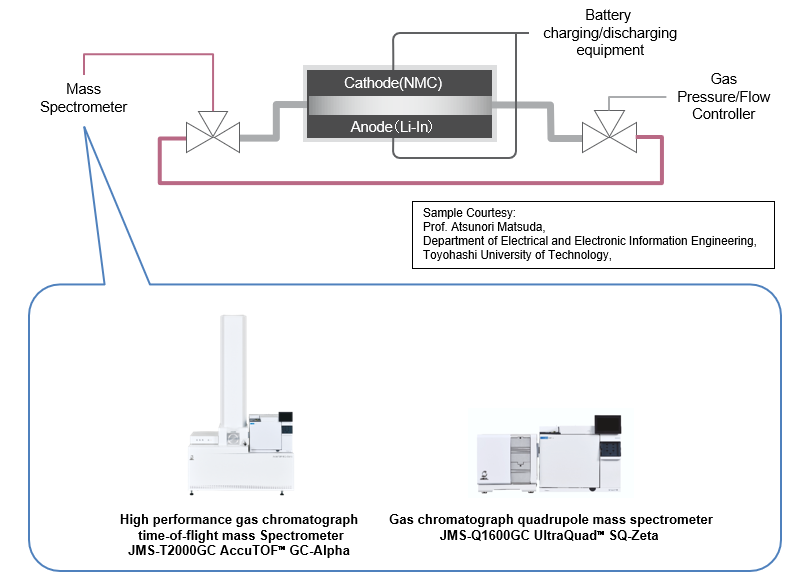
Identification of gas species generated inside a cell after charging and discharging using the JMS-T2000GC
The gases generated in the cell were measured with high mass resolution, and the extracted mass spectra of major peaks were subjected to compositional calculations using exact masses, as shown below. Various sulfides (H2S, CH4S, C2H6S), which are thought to originate from the solid electrolyte, as well as iodomethane (CH3I) and others were detected in the gas after charging and discharging. In addition, hydrogen sulfide (H2S) was detected among the components, indicating an increase in the amount of hydrogen sulfide generated after discharge.
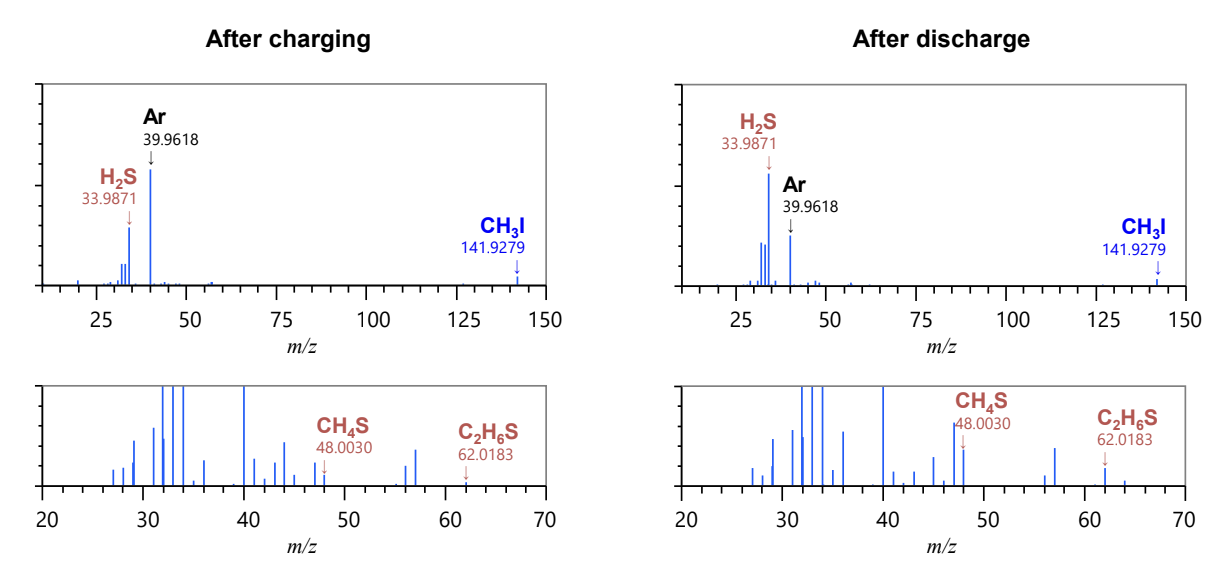
Changes in Gases Generated Inside a Cell During Charging and Discharging Using the JMS-Q1600GC
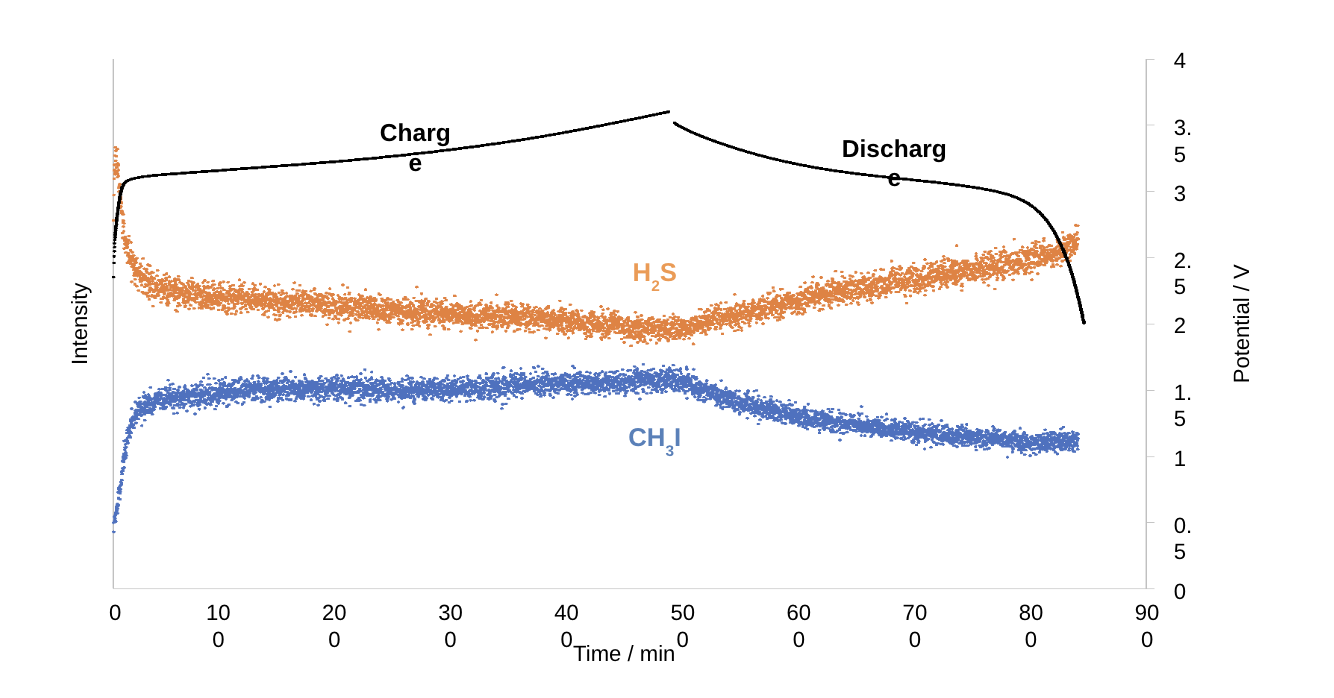
The results of real-time measurement of the gases generated inside the cell during charging and discharging are shown below. The graph shows changes in the amount of hydrogen sulfide and methane iodide generated inside the cell. For hydrogen sulfide, there is a large decrease immediately after the start of charging, followed by a gradual decrease and then an increase when the cell is switched to discharge. For iodomethane, the amount of iodine increases sharply immediately after the start of charging, then increases gradually, and then decreases when the cell is switched to discharge, showing the opposite behavior to that of hydrogen sulfide.
Acknowledgements
The authors would like to thank Prof. Atsunori Matsuda and Ms. Reiko Matsuda, Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, for providing samples for the preparation of this MSTips.
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
