Generated gas analysis of styrene-butadiene rubber using high-resolution TG-TOFMS (2)
MSTips No. 344
Introduction
Styrene-butadiene rubber (SBR) is used in a variety of products, including automotive tires, industrial products, and negative electrode binder materials for lithium batteries. Many synthetic rubber products, including SBR, contain various additives such as vulcanization accelerators, and information on the gases produced by heating is extremely important for evaluating product properties, quality, and safety. Thermogravimetric-Mass Spectrometry (TG-MS) is effective for the analysis of synthetic rubber because it is possible to correlate and obtain on the mass change and qualitative information on the generated gas when heating is applied. On the other hand, since this method does not use chromatogram separation, many rubber pyrolysis products (mainly hydrocarbons) make it difficult to analyze trace components. Therefore, mass separation by high-resolution TOFMS JMS-T2000GC was performed and useful results were obtained. Results using the EI method are reported in MSTips 343. In this report, we describe a highly sensitive negative ion measurement method by TG-MS using the Electron Capture Ionization (ECI) method. In addition to selective detection of molecular ions, which is a feature of the soft ionization method, the ECI method can selectively detect oxygen, sulfur, and various halogen compounds with high electronegativity, a feature of the negative ionization method. Therefore, the ECI method is considered to be effective for the analysis of trace constituents.
Experiment
Commercially available SBR rubber was used for the sample. A STA2500 Regulus manufactured by NETZSCH was used for TG, and the gas generated by heating was injected into the JMS-T2000G for analysis (Figure 1). The ECI method was used as the ionization method for the JMS-T2000GC, and the reagent gas Ar was introduced from the TG furnace. Table 1 shows the TG-MS measurement conditions.
Table 1. Measurement conditions
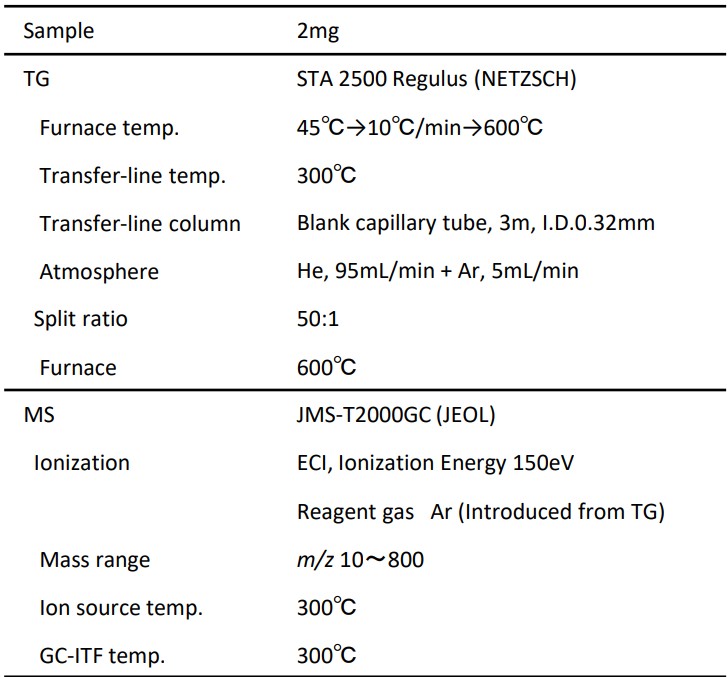

Figure 1. JMS-T2000GC with TG
Results and Discussion
Figure 2 shows the TG curves of SBR rubber during EI and ECI measurements. In the ECI method, the reagent gas Ar was introduced from the TG, but no significant change was observed in the TG curve. It was confirmed that there was no significant effect on the thermal reaction of SBR rubber in the TG furnace.
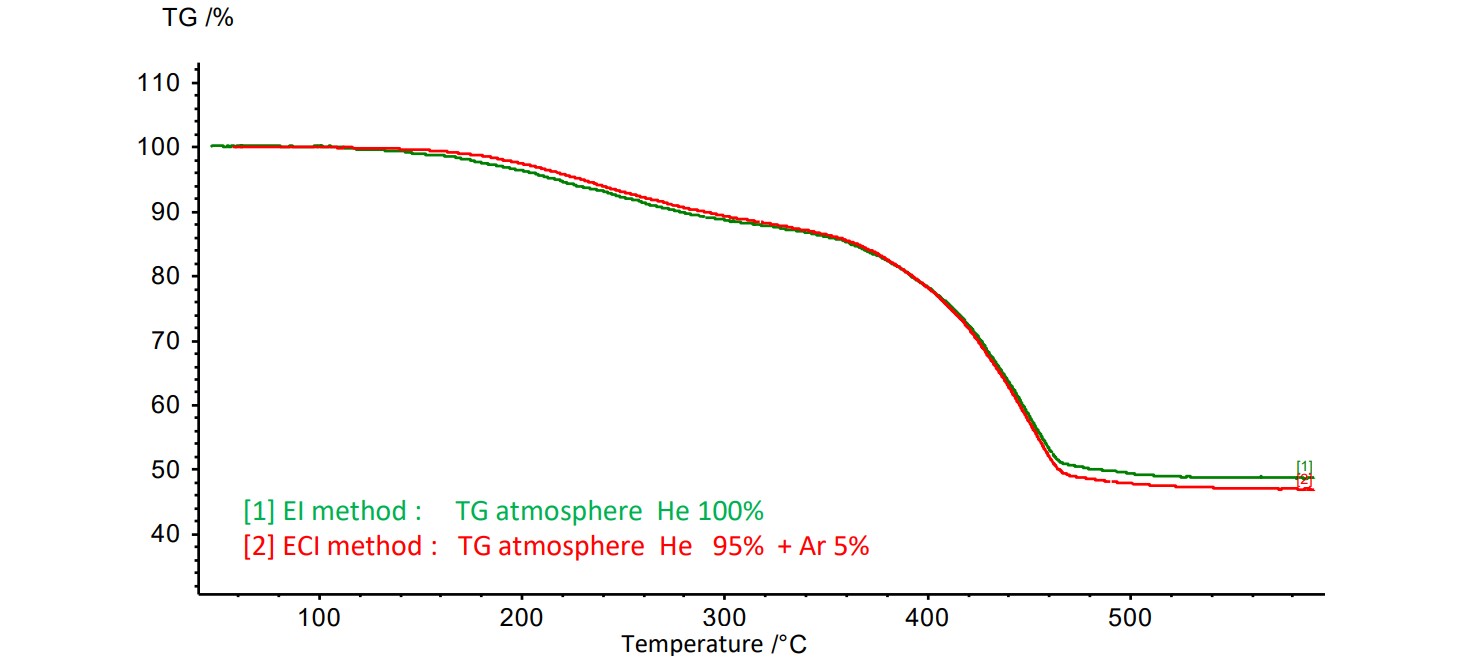
Figure 2. TG curves
Figure 3 shows the TIC chromatograms in the EI and ECI methods. Significant differences were observed in the peak shapes of both. The most notable difference was the 2-Mercaptobenzothazole peak, a sulfur compound around 350°C in area C of the ECI method.
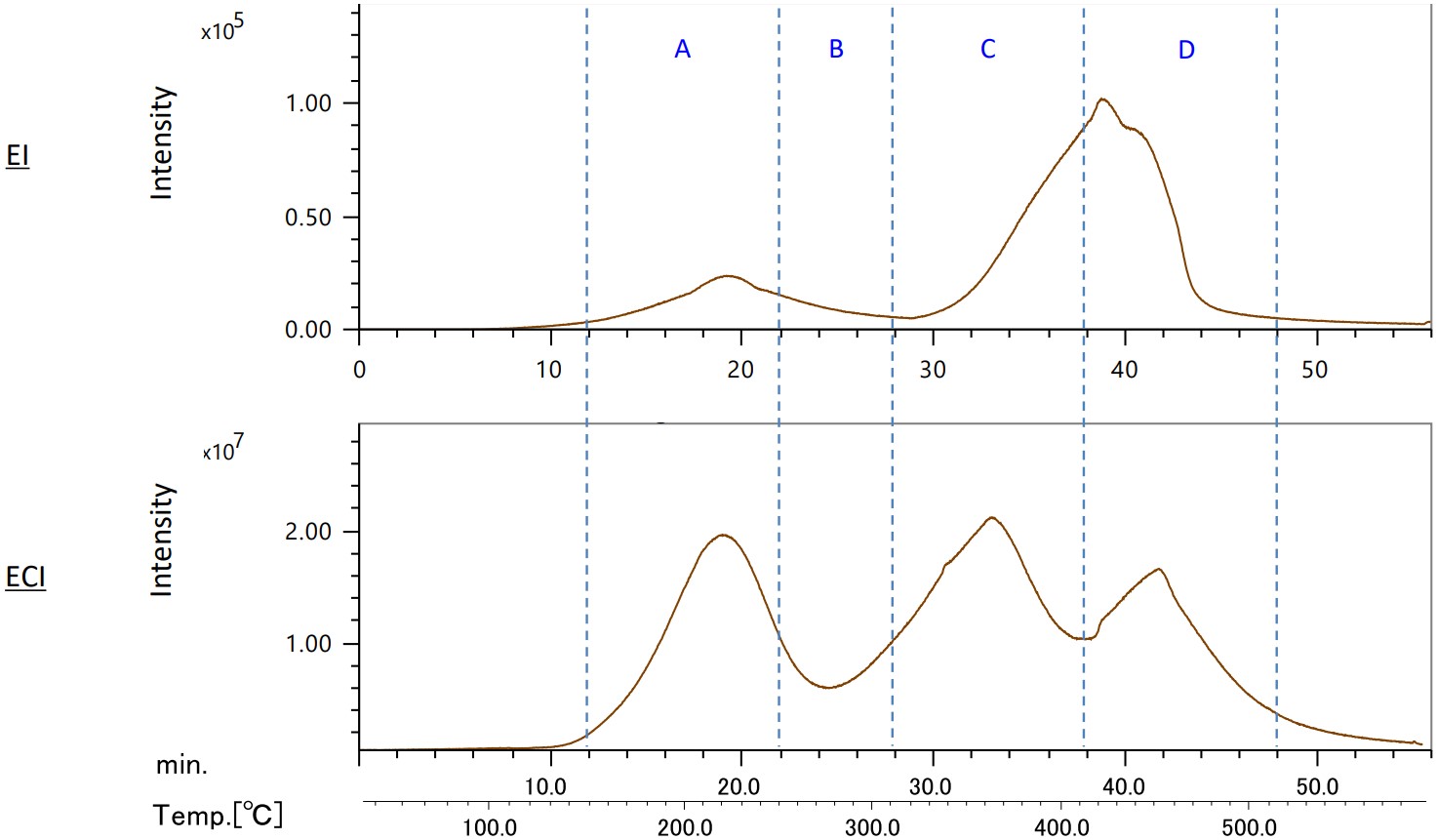
Figure 3. TICC in EI and ECI methods
Figure 4 shows the mass spectra of the EI and ECI methods in areas A-D on the TIC chromatograms. In areas B and C, the intensity of the sulfur compound 2-mercaptobenzotazole increased dramatically. This shows that the ECI method is effective for selective detection of sulfur compounds.
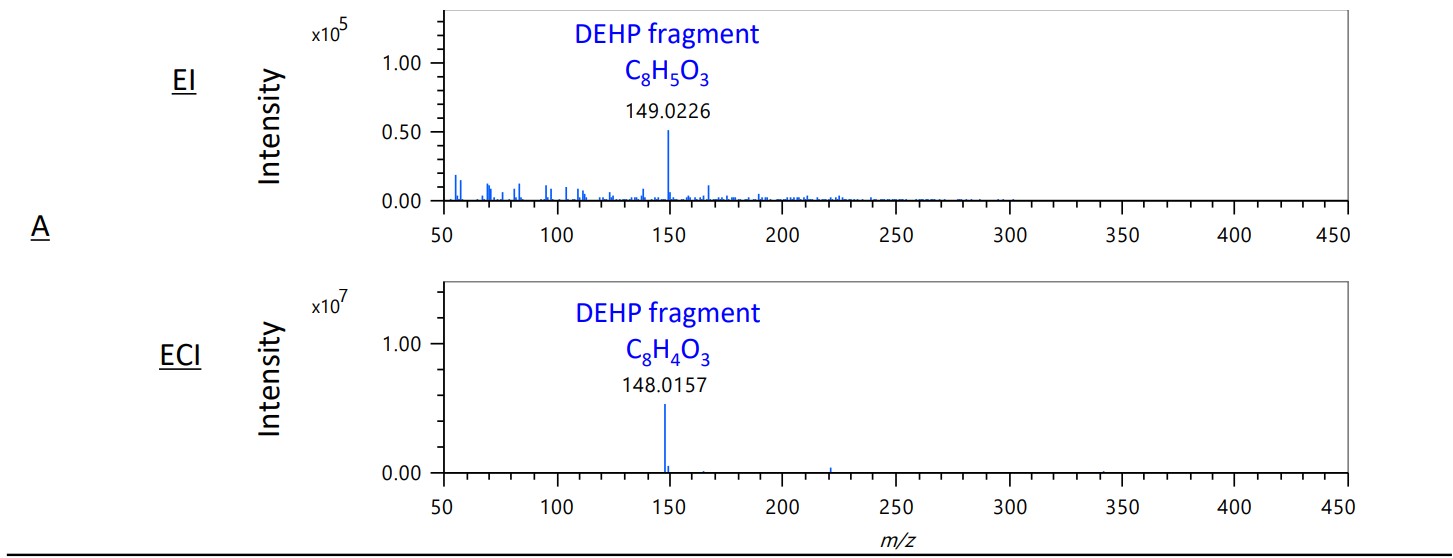
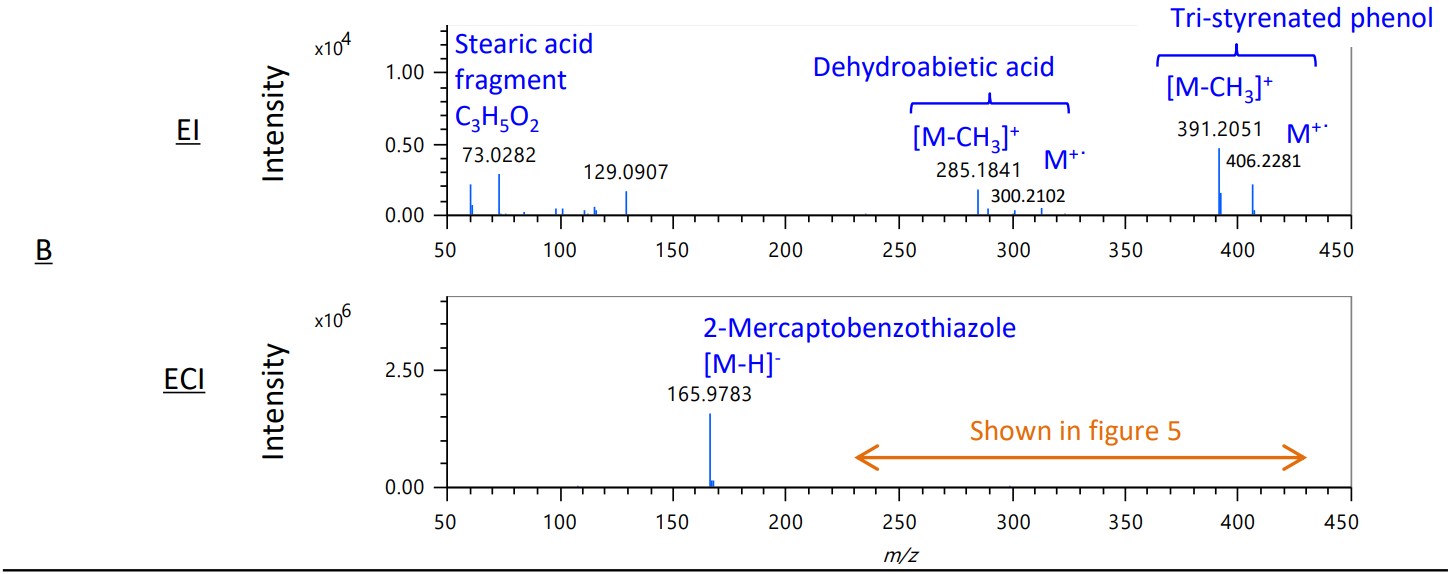
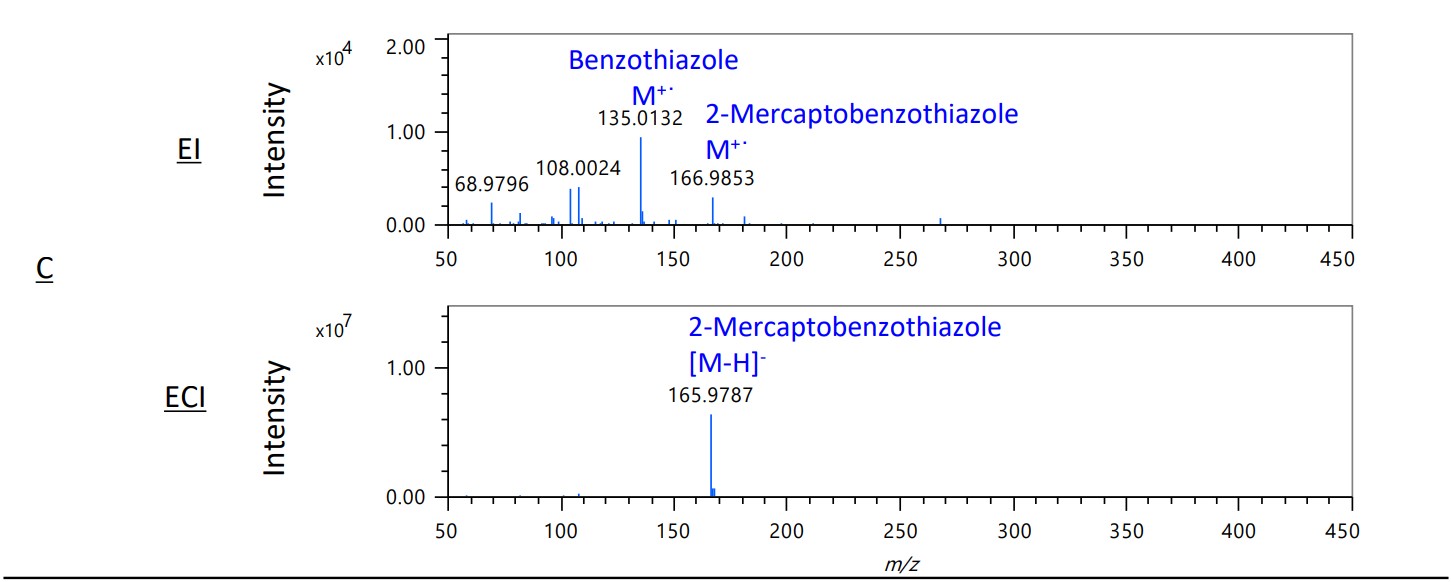
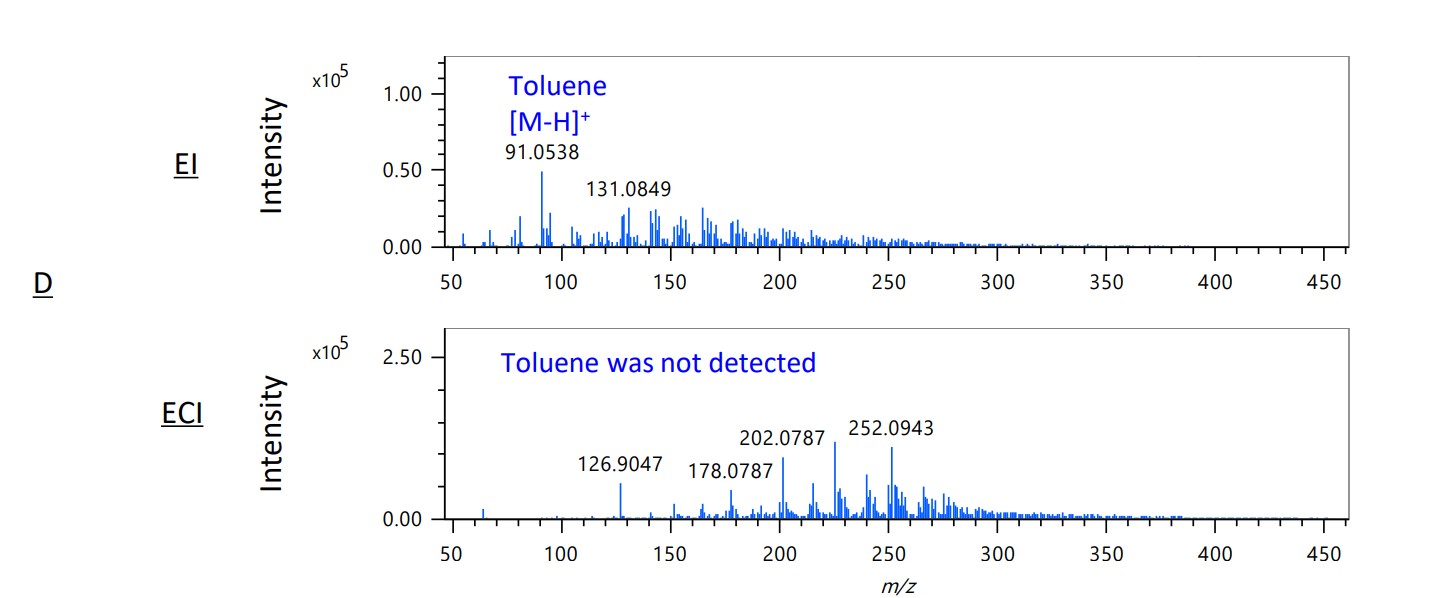
Figure 4. Mass spectra of EI and ECI methods in area A-D on each TICC
Figure 5 shows the expanded mass spectrum from m/z 230 to m/z 430 of area B in the ECI method. In addition to Dehydroabietic acid, a rosin component used as an anti-aging agent, which was also detected by the EI method, Abietic Acid and Dihydroabietic acid were detected in the ECI method. In the EI method, only the fragment ion m/z 73 of the fatty acid palmitic acid used as a builder was observed, while in the ECI method, [M-H]- was observed as well as stearic acid.
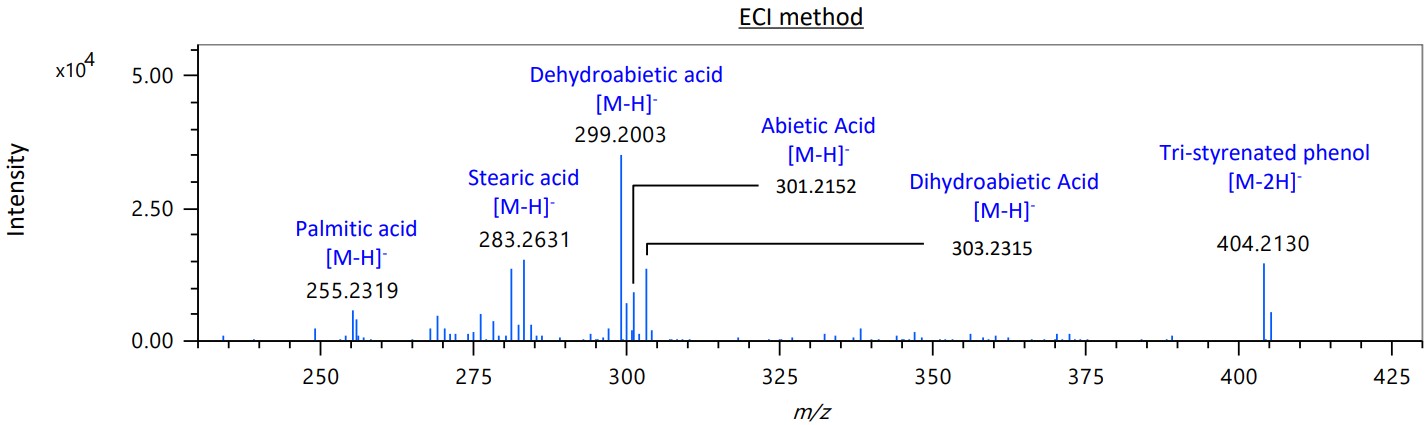
Figure 5. Mass spectrum (m/z 230-430) of area B on TICC
Figure 6 shows TIC chromatograms and extracted ion chromatograms (EIC) for the EI and ECI methods. Some components, such as Tri-styrenated phenol and 2-Mercaptobenzotazole, were observed over a wider temperature range by the ECI method than by the EI method. This shows that the ECI method can be used for confirmation the temperature at which trace constituents are generated.
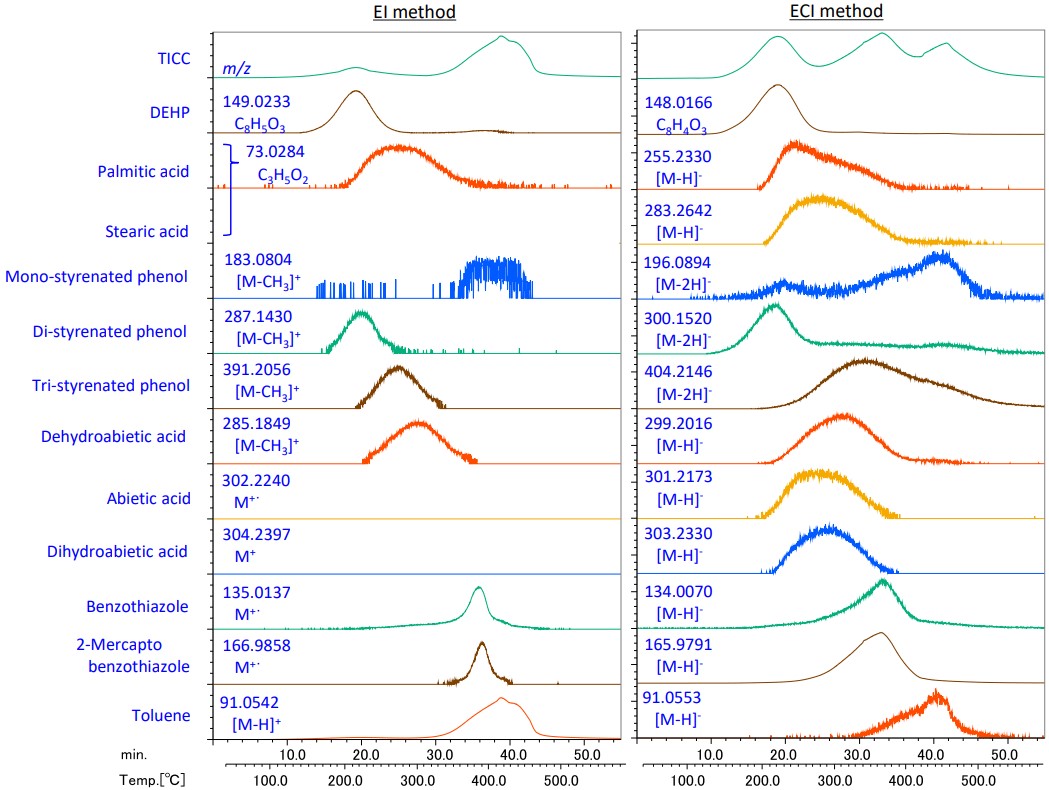
Figure 6. TICC and EIC (m/z±0.01Da) in EI and ECI methods
Figure 7 shows the EICs of sulfides and halogens. The effectiveness of the ECI method was confirmed because components not observed by the EI method were detected by that.
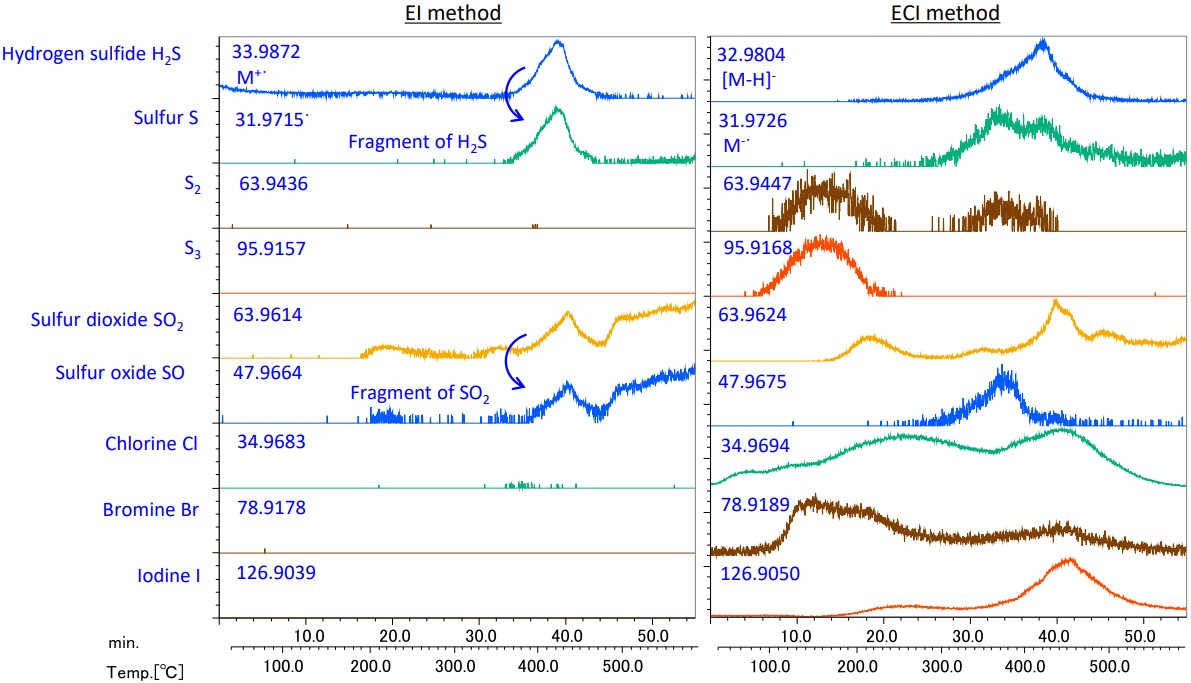
Figure 7. EICs (m/z±0.01Da) of Sulfides and halogens
Conclusion
The ECI method by TG-MS was confirmed to be effective for SBR rubber analysis. Since this method can selectively detect sulfur and halogen compounds that are concerned about their effects on the human body, it can be used to evaluate the safety of synthetic rubber and resin products.
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

