Elemental Composition Determination of Polymer End Groups Using Accurate Mass
MSTips No. 357
A molecular weight of a polymer species can be confirmed by combining soft ionization methods such as matrix-assisted laser desorption/ionization (MALDI) field desorption (FD), electrospray ionization (ESI), and a high resolution mass spectrometer capable of accurate mass measurements, such as a time-of-flight mass spectrometer (TOFMS). From the obtained mass spectrum, it is possible to identify polymer species (monomer identification), identify differences in end groups, and calculate the molecular weight distribution of each polymer series. Recently, it has been possible to efficiently overview the information in the mass spectra of complex polymer mixtures using a Kendrick Mass Defect (KMD) analysis software, msRepeatFinder. Determining the elemental composition of the end groups of the polymer series identified in this way is essential for confirming the state of polymer synthesis reactions, quality control of products, and analysis of degradation mechanisms. In this report, we introduce the procedure for determining the elemental compositions of the end groups from the accurate mass information of the polymer.
Procedure for determining the elemental composition of end groups
First, the primary structure of a polymer ion is shown (Figure 1). The structure of a polymer molecule consists of monomers, which are repeating units, polymerized for the degree of polymerization, and end groups (α terminal, ω terminal) are present at both ends. Depending on the measurement conditions, polymer ions observed on the mass spectrum are those to which adduct ions are added. The mass (Mn) of the polymer ion is:
Mn = Mr×n + Me + Mc (Mr: mass of monomer, Me: sum of the masses of both end groups, Mc: mass of adduct ion) (1)
Figure 2 shows the procedure for determining the elemental composition of both end groups. According to equation (1), in order to calculate "the total mass of both end groups and the adduct ion mass", the mass of the monomer multiplied by the degree of polymerization can be subtracted from the mass of the polymer ion. The mass of the monomer referred to here is the calculated mass of the monomer whose elemental composition is determined from the mass difference between two peaks that differ by one degree of polymerization. The calculated mass of the monomer should be used because the observed monomer mass (mass difference between the two peaks) contains an experimental error. If the monomer mass contains an experimental error, the experimental error is multiplied by the degree of polymerization and affects the estimation of the composition of the end groups. For example, a small experimental error of 1 mDa in the monomer mass results in a large error of 20 mDa when calculating the end group composition of the 20-mer polymer. Now, the mass obtained by subtracting the mass of the monomer times the degree of polymerization from the mass of the polymer ion is the sum of the masses of both terminal groups and adduct ions. However, the degree of polymerization cannot be determined from the accurate mass obtained from the mass spectrum. Therefore, in the procedure, a range is set for the total mass of the end groups and adduct ions, and in the process of repeatedly subtracting the monomer masses, the composition is estimated only when the mass falls within that range. Therefore, uncertainty remains in the composition estimation result by an integer multiple of the monomer composition.
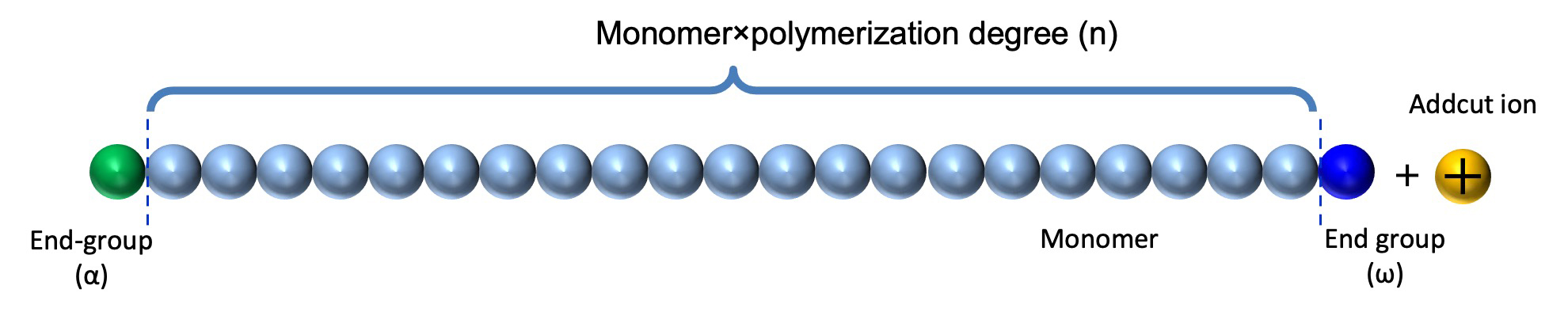
Figure 1 The primely structure of polymer ion
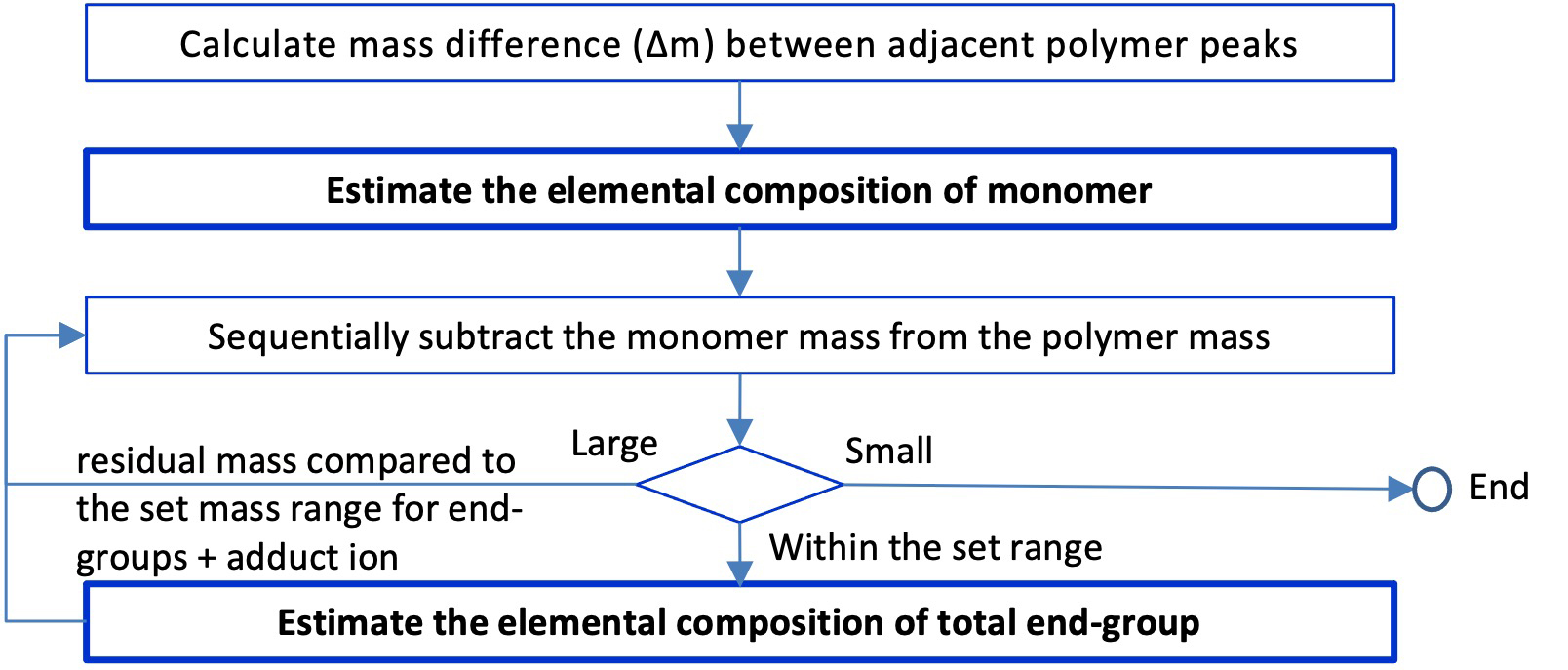
Figure 2 The procedure for composition estimation of polymer end-groups
Example of estimating the elemental composition of end groups
Here, the procedure for analyzing a mixture of polyethylene oxides with different end groups using msRepeatFinder V5.0 (the polymer composition estimation function was implemented from msRepeatFinder V4) will be explained. Figure 3 shows the mass spectrum, KMD plot and KMR (Kendrick Mass Remainder) plot. Since C2H4O was specified as the base unit, series of polyethylene oxides are arranged horizontally in the KMD plot. In the KMR plot, series with the same end group are aggregated at a single point, and five polyethylene oxide series with different end groups were confirmed in this mass spectrum. Each polymer series was grouped and color-coded.
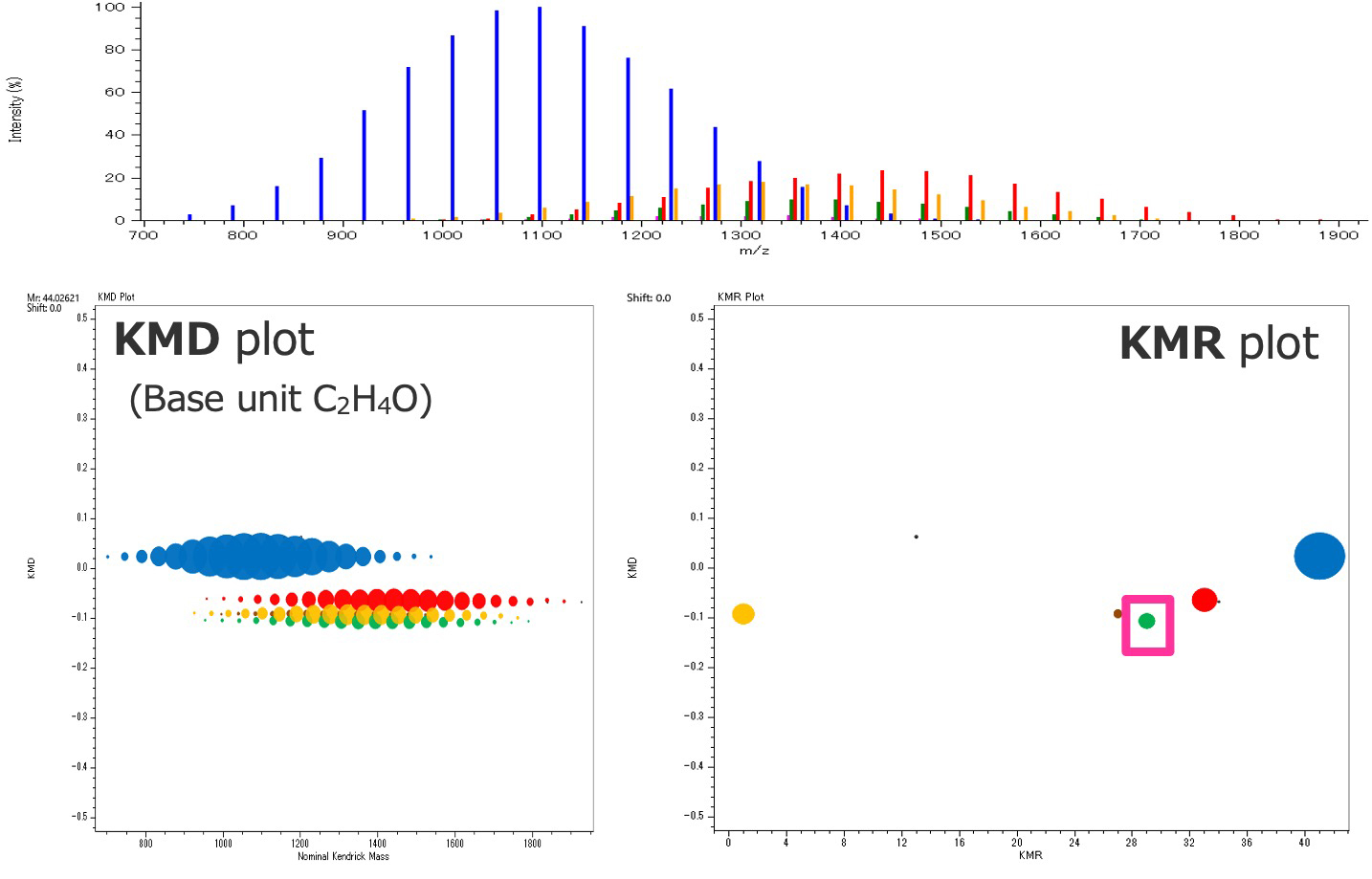
Figure 3 KMD/KMR plots of the mixture of polyethylene oxides
The procedure for estimating the composition of terminal groups is shown using the green series enclosed in the square in the KMR plot in Figure 3 as an example. First, if we select two peaks at 1261.85633 Da and 1305.88291 Da, which have one different degree of polymerization, as Peaks 1 and 2, the mass difference (Δm) of 44.02658 Da between the two peaks is automatically calculated (Figure 4 – 1.). The elemental composition of the monomer (neutral) is estimated from this mass difference. The conditions are the same as those for general ion elemental composition estimation; mass error tolerance, the number (even or odd) of electrons, the DBE, and the element information. Select one monomer elemental composition from the suggested estimation results (Figure 4 – 2.). This is because, as described above, the remaining mass obtained by repeatedly subtracting the calculated mass of the monomer from the observed mass of the polymer (peak 1) is used to estimate the elemental composition of the end group. Conditions for estimating the elemental composition of end groups differ from general conditions for estimating the elemental composition in that it is necessary to specify the "range of the sum of the masses of the terminal groups and adduct ion" in addition to the selection of the monomer composition. This is because, as mentioned above, the degree of polymerization cannot be determined from the exact mass, so the calculated mass of the monomer is repeatedly subtracted from the observed mass of peak 1, and composition estimation is performed only when the result falls within this mass range. The estimated elemental composition of the end groups is displayed together with the degree of polymerization and adduct ion (Figure 4 – 3.). When confirming the results of the elemental composition estimation of the end groups, four candidates are displayed, but they all have the same elemental composition of the polymer and differ in the degree of polymerization.
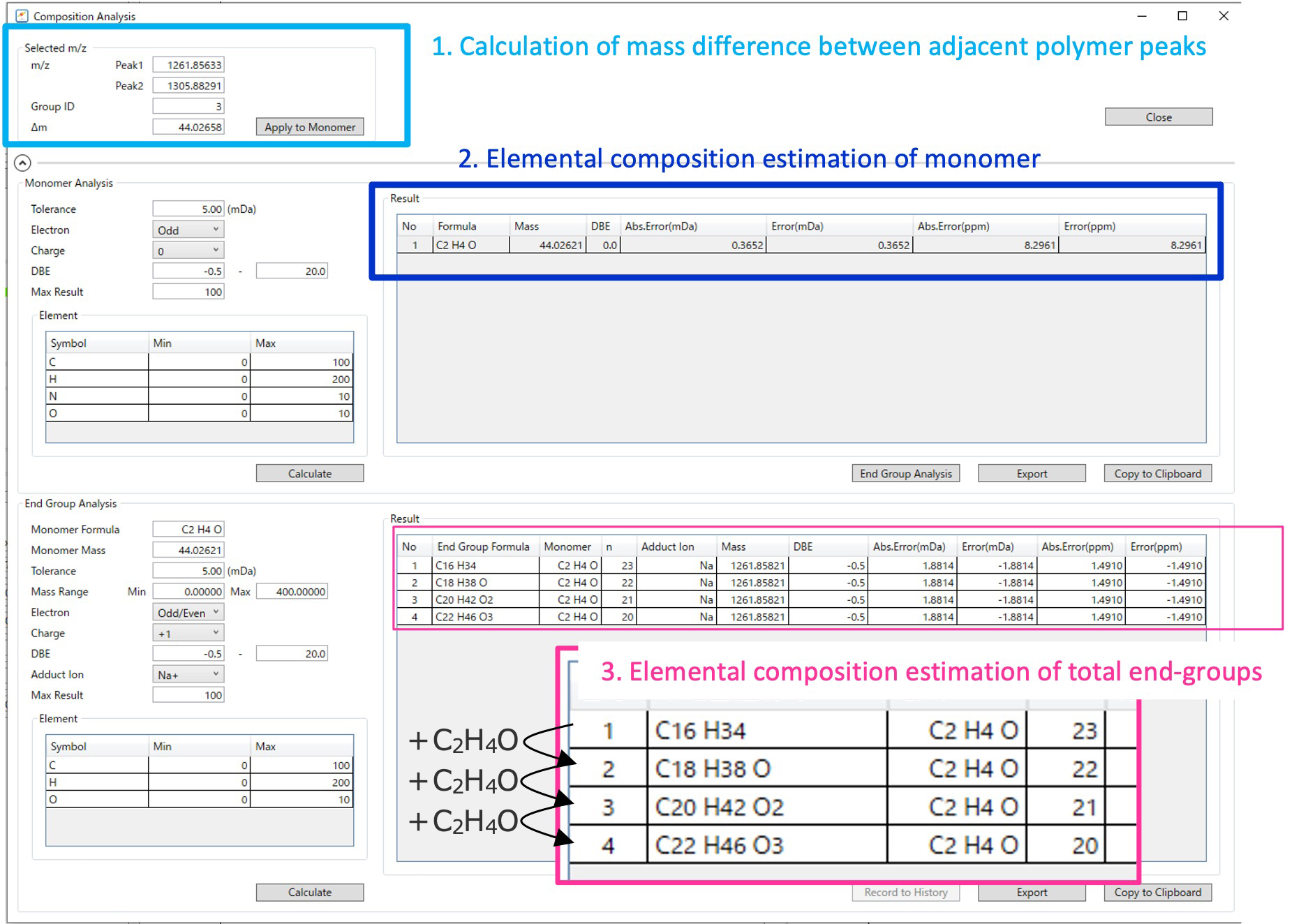
Figure 4 The GUI for elemental compositions estimation of end-groups in msRepeatFinder
Checking the elemental composition estimation history
In msRepeatFinder V5, composition estimation results can be saved in history (Figure 5). Select one of the end group composition estimation results and click "Record to History" to save the composition estimation result in the list. Displaying the saved results by selecting the "Composition Analysis History", the composition estimation conditions and composition estimation results can be checked. Furthermore, when you click the "Details" button, candidates other than the selected composition estimation result are displayed. If you want to change the result of the terminal group composition, you can change the content of the composition estimation history by specifying the line and clicking the "Change Endgroup Formula".
Summary
In this report, a method for estimating the elemental composition of polymer end groups using accurate mass is described. Since the primary structure of a polymer is composed of a combination of monomers and terminal groups, even if the mass of polymer ions is large, the target of composition estimation is a partial structure with a relatively small mass. Therefore, if the element information is known to some extent, it is easy to estimate the structure. On the other hand, since the exact mass alone cannot determine the degree of polymerization, and only the sum of both end groups can be obtained as a composition estimation result, it is important to have some prior information on the structure of the end groups, especially the range of their masses.
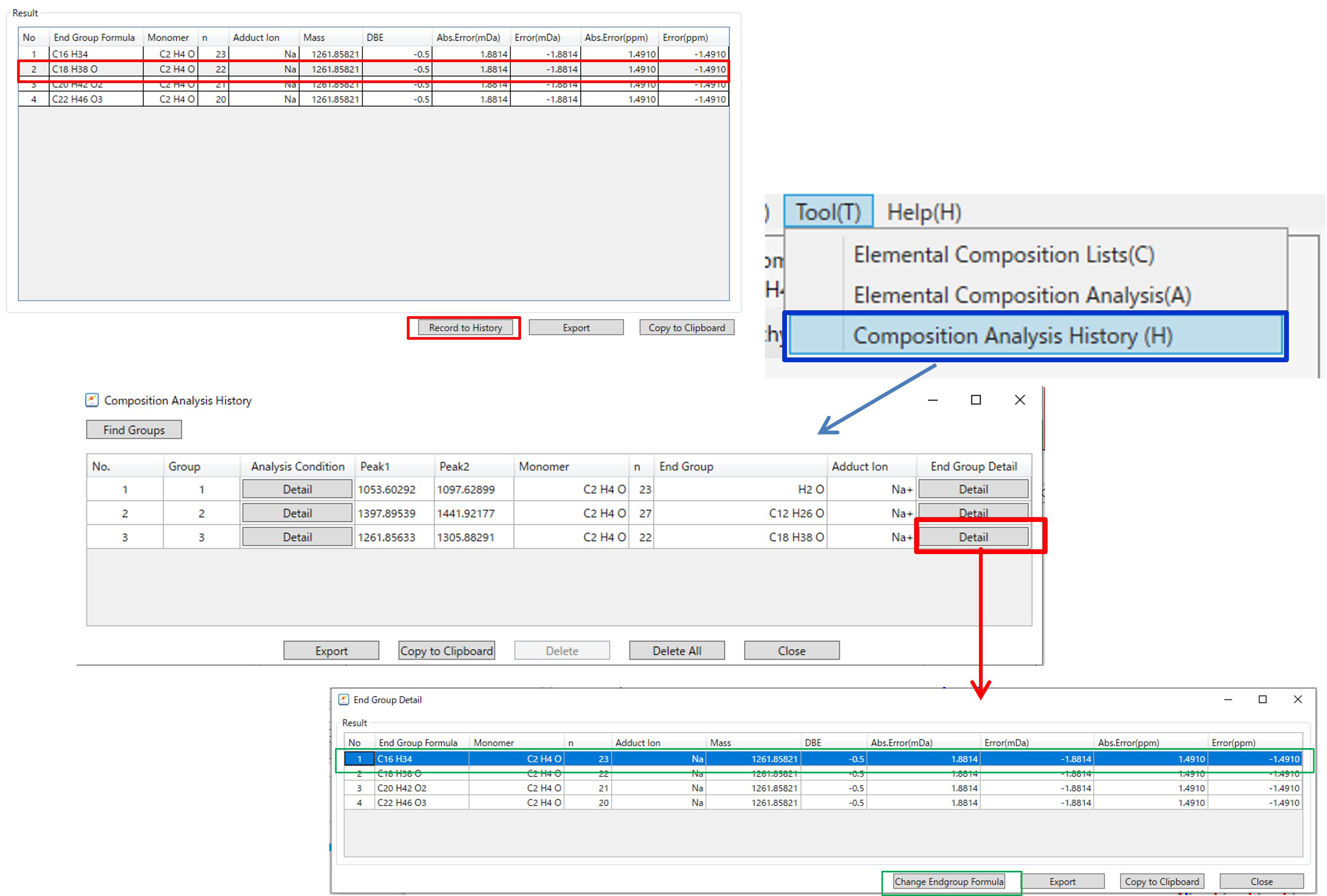
Figure 5 GUI for history of elemental composition estimations of polymers
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
