Application of MICCS-NMR #2 Kinetics analysis of radical addition
NM060008E
Reaction
This note treats triethylborane (Et3B)-mediated radical addition to oxime ether, where a borane complex is proposed as a key intermediate. However, its isolation is very difficult using conventional methods.
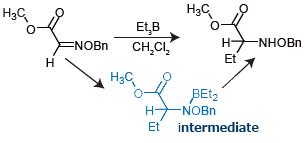
Sample is by courtesy of Prof. T. Naito, Prof. O. Miyata,
and Dr. M. Ueda of Kobe Pharmaceutical University.
Protocol of NMR measurements
Information on reaction rate can be obtained by changing time required for the sample in MICCS to move from the reaction point to the detection point. The example shown below demonstrates that, by lowering the entire flow rate with keeping flow ratio of oxime ether and Et3B to be 1 to 1, NMR signals of reactants gradually decrease while those of intermediate increase.
| 0.5M oxime ether | 0.5M Et3B |
| 20.0 | 20.0 |
| 10.0 | 10.0 |
| 5.0 | 5.0 |
| 2.0 | 2.0 |
| 1.0 | 1.0 |
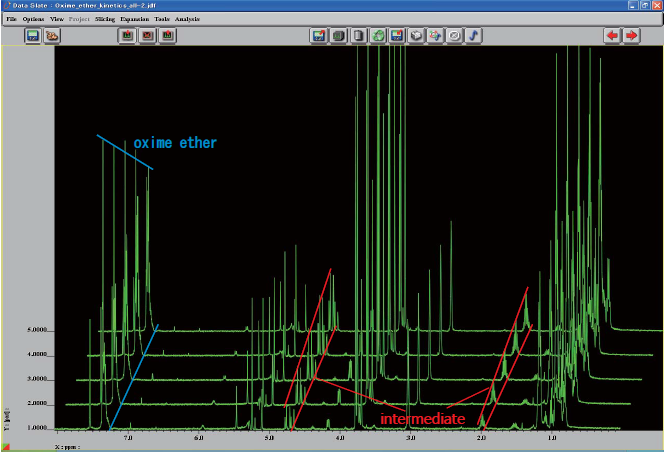
※ CH2Cl2signal is suppressed.
Spectrometer: JNM-ECA500
Accumulation: 16scan
Recycle delay: 10s
SEARCH APPLICATIONS
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
