Measurement of Vitamin C/ Ascorbate Radical by Using ESR
ER120001E
Vitamin C (V.C)/ L-Ascorbic Acid(Fig. 1(a))is one of the vitamins which are most-studied in recent years. It is known as a water-soluble antioxidant and it is also used as an additive for foods. It can be auto-oxidized easily to give Dehydro-ascorbic acid (DHA) radical.
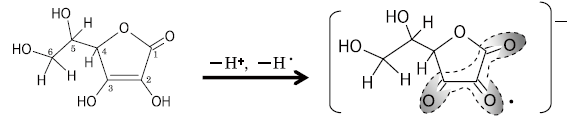
The V.C. aqueous solution (1 Mol/L) was measured after N2-gas bubbling for 30 seconds. The result is shown in Fig. 2. For DHA radical, the unpaired electron is delocalized on the carbonyl groups C1 to C3, and it is expected to have considerable spin distribution on oxygen atoms 1).
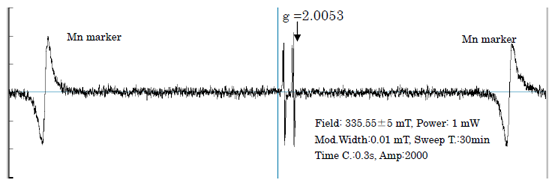
Fig.2 ESR Spectrum of V.C Radical
The ESR signal of DHA radical is recorded in precise in Fig. 3. The hyperfine couplings, a4H=0.18mT and a6CH2=0.019mT, are typical for DHA radical.
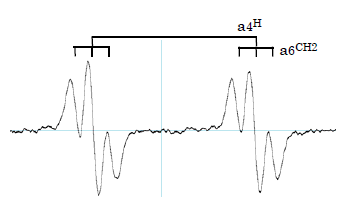
Fig. 3 ESR signal of DHA radical
It is expected that quantity of DHA radical indicates the redox condition around the radical.2).
Reference
1) G.P.Laroff et.al. J.Amer.Chem.Soc. 94 9062-9073 1972
2) G.R.Buettner et.al. Free Radical Biology & Medicine 14 49-55 1993
SEARCH APPLICATIONS
Related Products
Solutions by field
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
