Structural analysis of a small molecule using JMS-S3000 "SpiralTOF™-plus 2.0" ー MS/MS measurement of a photodegradation product of reserpine ー
MSTips No. 365
High mass-resolution MALDI-TOFMS can determine the elemental compositions of target compounds from their measured accurate masses. MS/MS measurement is effective in elucidating the chemical structure, which is difficult to infer from accurate mass information. MS/MS measurement using JMS-S3000 "SpiralTOF™-plus 2.0" with a TOF-TOF option can detect fragment ions generated by high-energy collision-induced dissociation(HE-CID). HE-CID is a technique of fragmenting precursor ions by a single collision. It is known to provide more structural information than low-energy collision-induced dissociation, which fragments precursor ions by multiple collisions. On the other hand, since MALDI-TOFMS is difficult to connect online with pre-separation methods such as liquid chromatography, the target compounds must be separated by only their masses. High precursor ion selectivity is required to obtain accurate structural information for components that are close in their masses. SpiralTOF™-plus 2.0 with TOF-TOF option can achieve high precursor ion selectivity due to the long flight path of the spiral ion optics used in the 1st TOFMS. In this application note, we report the structural elucidation of a photodegradation product of reserpine as an example of structural analysis of a component close to a possible interference in mass by MS/MS measurement using the SpiralTOF™-plus 2.0 with TOF-TOF option.
Measurement conditions
A reserpine solution was exposed to sunlight in a room for a few days. Sample preparation method and measurement conditions were shown in Table 1. The mixture of sample and matrix solutions were spotted on a target plate and air-dried. The MS and MS/MS measurements were performed on the same sample spot.
Table 1. Sample preparation and measurement conditions
| Sample | Reserpine, 1mg/THF solution |
|---|---|
| Degradation method | Daylight (A few days, Indoor) |
| Matrix | DHB, 10mg/mL THF solution |
| Spotting method | Sample + Matrix 1:10 mixed solution was spotted on the target plate |
| Mass spectrometer | JMS-S3000 SpiralTOF™-plus 2.0 |
| Measurement mode | Exact mass measurement: Spiral mode MS/MS measurement: TOF-TOF mode |
| Mass calibrant | PPG 700, 10mg/mL THF solution |
Results
Figure 1 shows measurement results in spiral mode. Protonated molecules [M+H]+ of reserpine and its photodegradation product were detected at m/z 609 and m/z 607, which are 2 u apart, respectively. MS/MS measurements were performed for m/z 607 and m/z 609, and product ion mass spectra shown in figures 2a and 3a were obtained. Na+ or K+ were not present in both product ion mass spectra, confirming that the precursor ions were [M+H]+. Applying the adduct ion information to elemental composition estimation conditions, m/z 609 and 607 were matched to reserpine and dehydrogenated reserpine, respectively (Figures 2b and 3b). Furthermore, it was surmised that the double bond position in the photodegradation product of reserpine was located in the heterocyclic portion (Figures 2c and 3c).
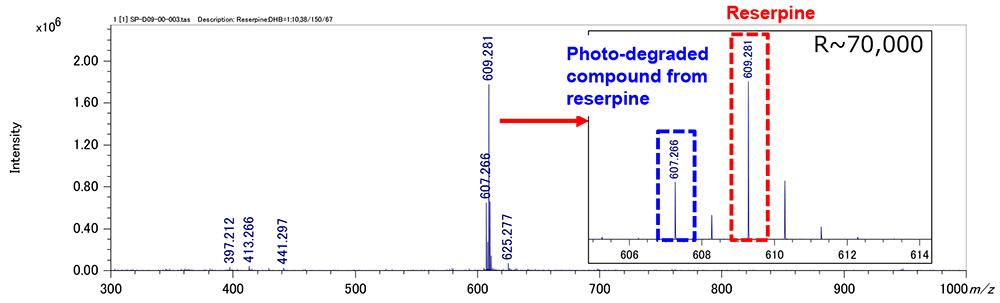
Figure 1 Mass spectrum of reserpine and its photo-degraded compound (Spiral mode)
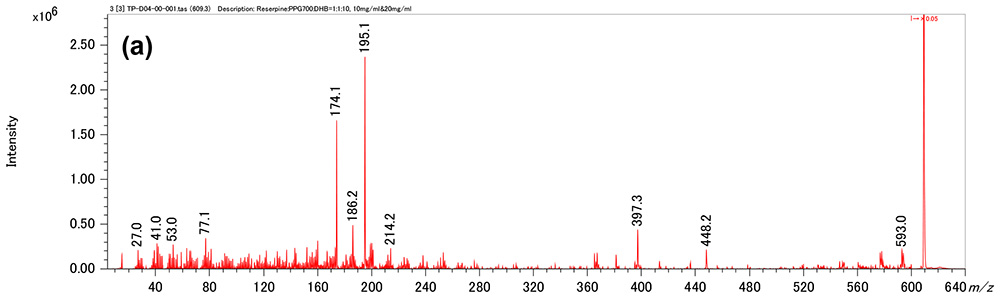
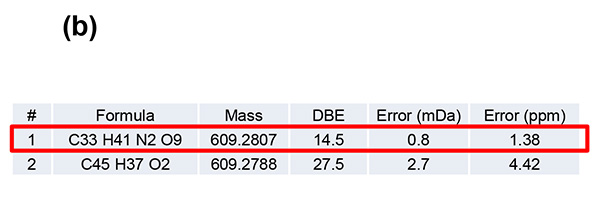
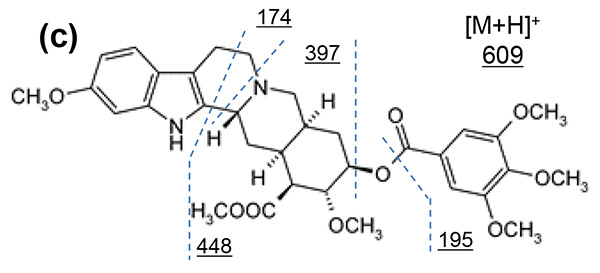
Figure 2 Product ion mass spectrum of reserpine (a). Inferred elemental composition of the ion (b) and estimated fragmentation channels(c).
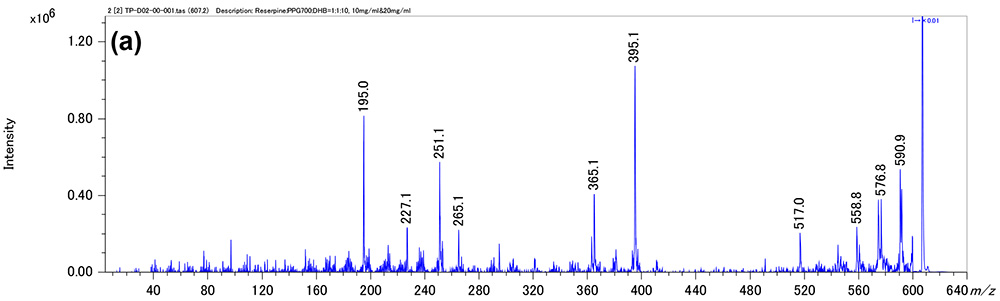
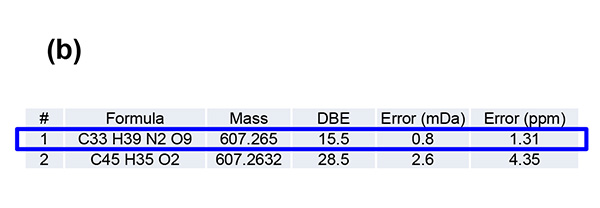
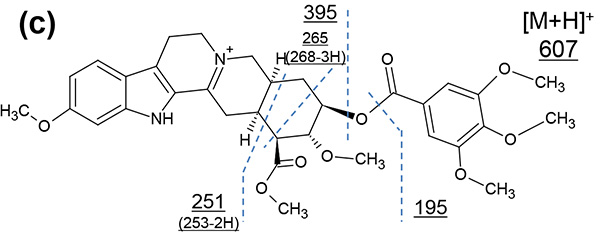
Figure 3. Product ion mass spectrum of the photodegradation product of reserpine (a). Inferred elemental composition of the ion (b) and estimated fragmentation channels(c).
Conclusion
SpiralTOF™-plus2.0 with TOF-TOF option has the high precursor ion selectivity so that the product ion mass spectra of reserpine and its photodegradation products, which differ only 2 u, were obtained separately. Adduct ions can be identified from the product ion mass spectra, and thus, it was possible to narrow down the candidates of elemental compositions. The obtained product ion mass spectra were significantly different between the two, and their chemical structures can be elucidated from unique product ion mass spectra.
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 698.8 KB
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
