Rapid Confirmation of Synthetic Compounds Using DART™ –Direct Mass Spectrometric Analysis from NMR Sample Tubes–
MSTips No.223
Introduction
The DART™ (Direct Analysis in Real Time) ion source was conceived at JEOL USA, Inc. in 2003 as a pioneer of the “ambient ionization” that ionizes a sample under atmospheric pressure and performs direct mass spectrometric analysis. After various developments and improvements, it was launched in the market in 2005. Today, many applications using the DART™ ion source have been published, and the number of related papers exceeds 400.
Since the DART™ ion source enables analysis in an open air under atmospheric pressure, it can handle a wide range of samples such as solids, liquids, and gases. In addition, since the measurement is only to hold the sample over the gas flow expelled from the DART™ ion source, anyone can easily and quickly perform the measurement. Many applications have been published that utilize these characteristics of DART to quickly determine the elemental composition of organic compounds. This time, we report an application related to synthetic compound confirmation that combines nuclear magnetic resonance(NMR), which is indispensable for structural analysis of organic compounds, and mass spectrometry by DART.
Results and discussion
Dulcine (C9H12N2O2) was used as the sample, and DMSO and deuterated DMSO (DMSO-d6), which are often used in NMR, were used as the solvents. Samples were prepared at 20 mg / 0.6 mL for both DMSO solution and DMSO-d6 solution.
Fig. 1 shows the sampling method from the NMR sample tube. For sampling, an elongated glass rod was prepared and used. The measurement was carried out by immersing the tip of the glass rod in the sample solution in the NMR sample tube and holding it directly over the DART ion source. The measurement was completed in less than a minute.
Fig. 2 shows the DART mass spectrum of Dulcine in DMSO solution and DMSO-d6 solution. In DART, water molecules in the atmosphere are first ionized, and the sample molecules are ionized by the ion-molecule reaction between the water cluster ions and the sample molecules. Since hydrogen and deuterium contained in the solvent do not affect the ionization, protonated molecules ([M + H] +) are observed regardless of the composition of the solvent. As a result, the isotope peak pattern of the observed Dulcine protonated molecule agreed well with the theoretical isotope pattern. At the same time, an accurate mass analysis was carried out, and the elemental composition was confirmed with a good mass accuracy of 1.1 mDa or less.
Since DART is not affected by deuterated solvents, it is possible to quickly and easily determine the molecular weight of the compound and elucidate the elemental composition of the molecular.
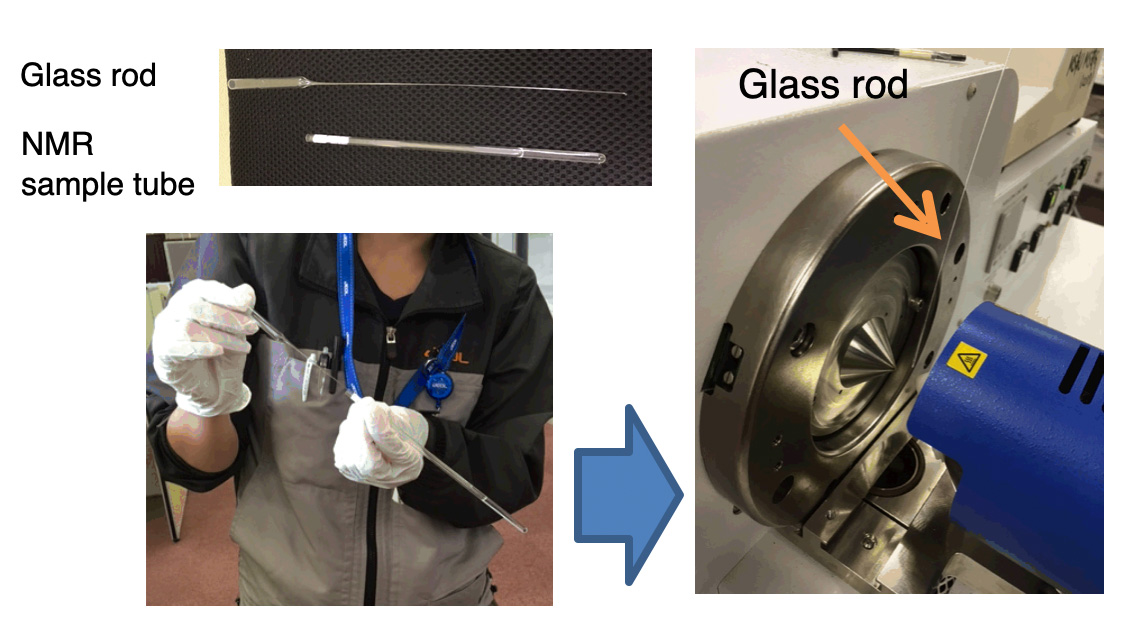
Fig.1 DART sampling
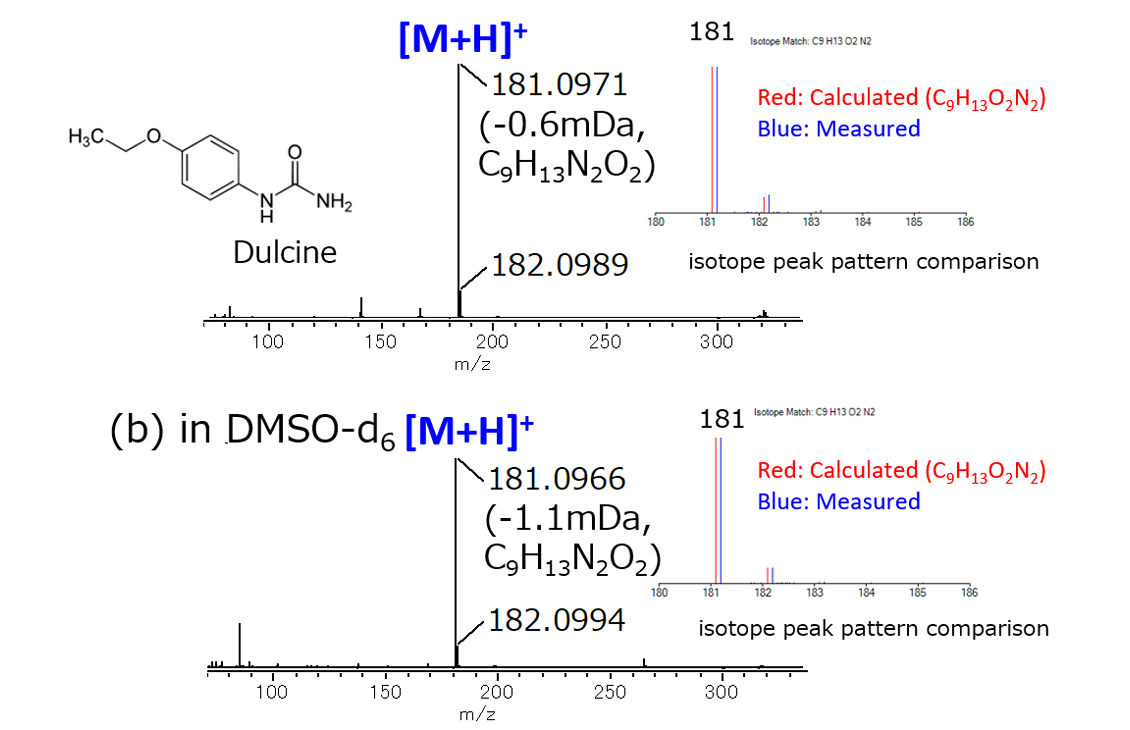
Fig.2 DART mass spectra of Dulcine
(a) in DMSO, (b) in DMSO-d6
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 1,228.8KB
Solutions by field
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
