

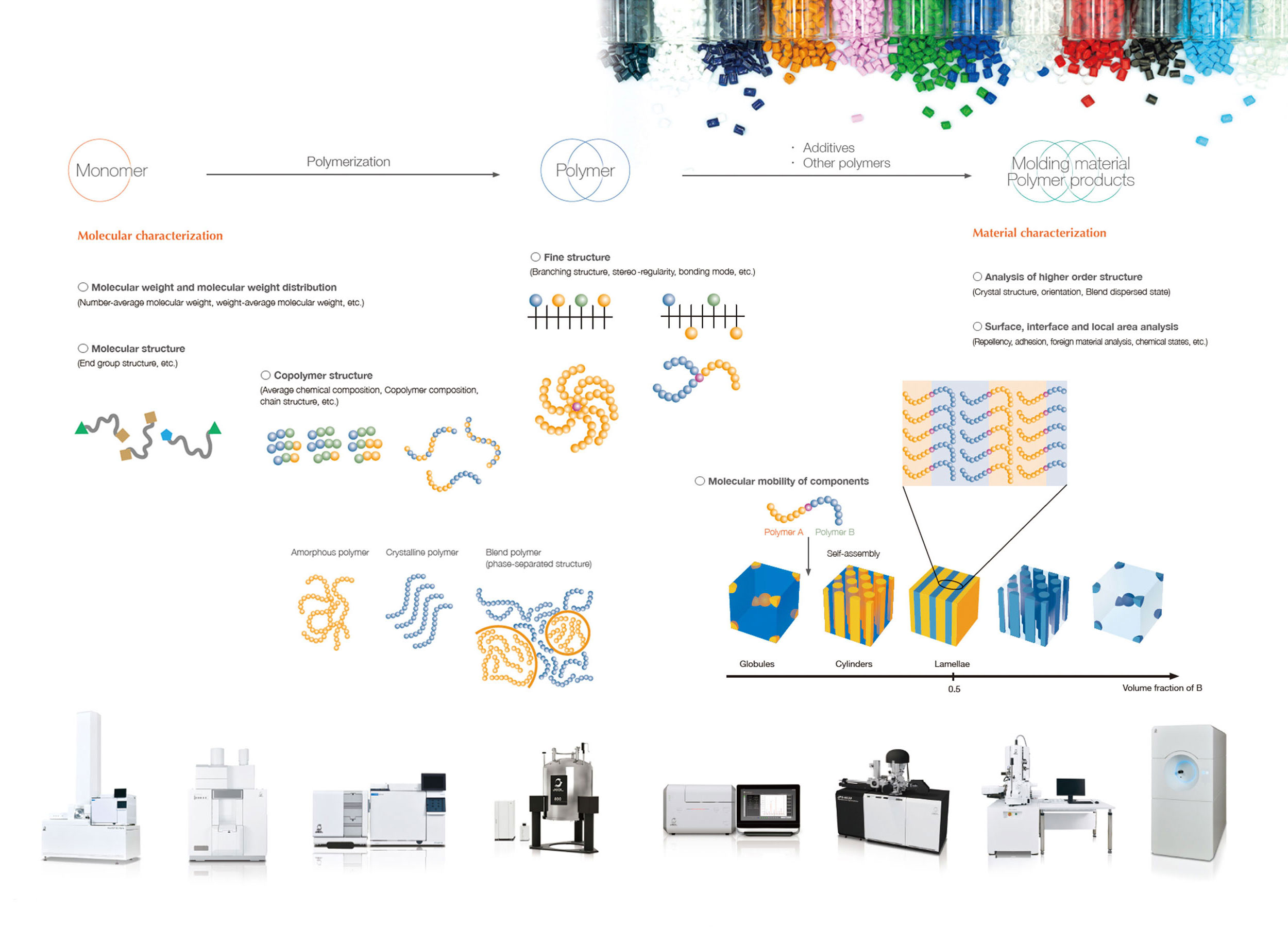
Plastics/Polymer
Polymers are used in a wide range of fields such as food wrapping materials, medicine packing, and industrial materials and products. Properties and functionalities of polymer materials are dependent on various factors, including molecular weight, molecular weight distribution, molecular chemical structures (primary structures), morphology of molecular chains (secondary structures) depending on the spinning angles ofchemical bonds, and crystalline or non-crystalline structures of inner-molecules and inter-molecules, as well as spherulite structure aggregates, phase separation structures and orientations (higher-order structures).It is of prime importance to analyze and evaluate the structures and properties of polymers as well as the correlation between these two factors, and to feed these analysis and evaluation results to the development and manufacturing sites for the enhancement of the performance and quality control of polymer materials and polymer products. On the other hand, industry in Japan is supported by functional molecules, which are becoming more advanced and more complicated. This makes it more difficult to characterize such polymers, there by increasing the importance of multiple analyses that combine a variety of analytical methods.
This page introduces a wide range of instruments used for polymer analysis and their applications.
Analysis of Various Polymer Materials
Analysis of PET Bottles for Outdoor Exposure Testing
Test method
Conforms to JIS Z 2381 (General Requirements for Atmospheric Exposure Testing)
Type of test: Direct exposure
Exposure angle: 20 degrees south face
Place of testing: Miyakojima, Okinawa
Sampling period: 0.5, 1, and 2 years
Start date: November 2020

Acknowledgements: Samples were provided by courtesy of the Council for PET Bottle Recycling for the degradation evaluation of PET bottles through outdoor exposure testing.
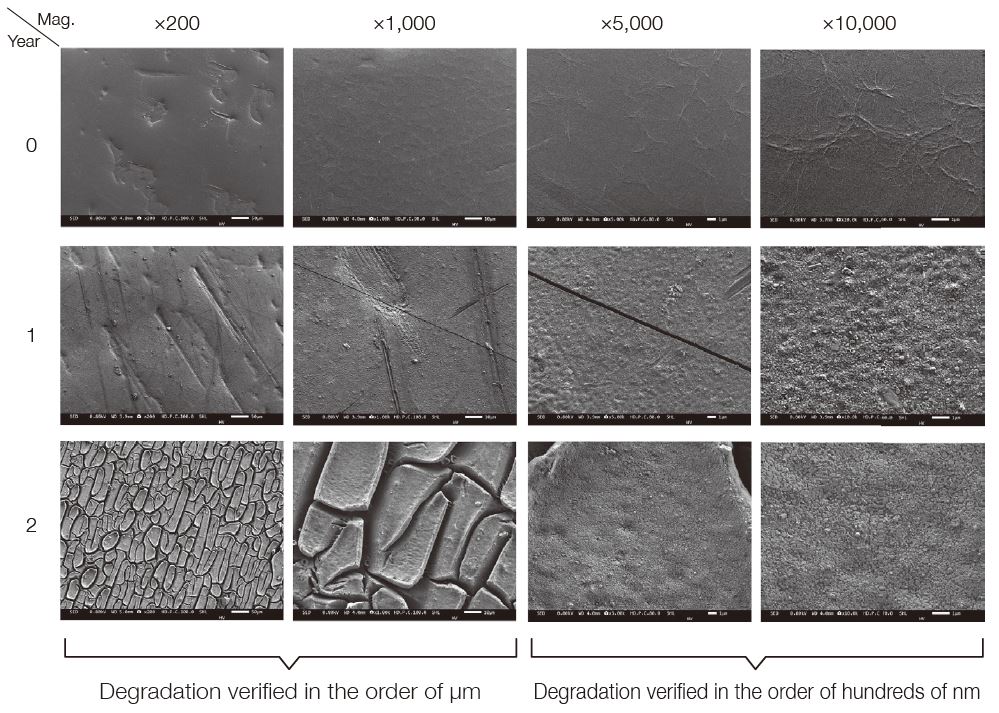
Morphological Observation of PET Surface Using SEM
By using SEM, new PET surfaces and surfaces exposed to outdoor atmosphere for 1 and 2 years were observed at various magnifications. Since SEM uses electron beams as the irradiation source, the influence of charge must be considered when observing highly insulating polymer surfaces. However, by observing at low incident voltage, the charge on the sample surface can be reduced, and the morphology of the polymer surface can be visualized without a conductive coating. In addition, by acquiring the secondary electron images of the surface, it is possible to obtain an image with a large focal depth, which is difficult to obtain with an optical microscope as well as observe the microstructure of the surface in detail.
Sample: Surface of PET film
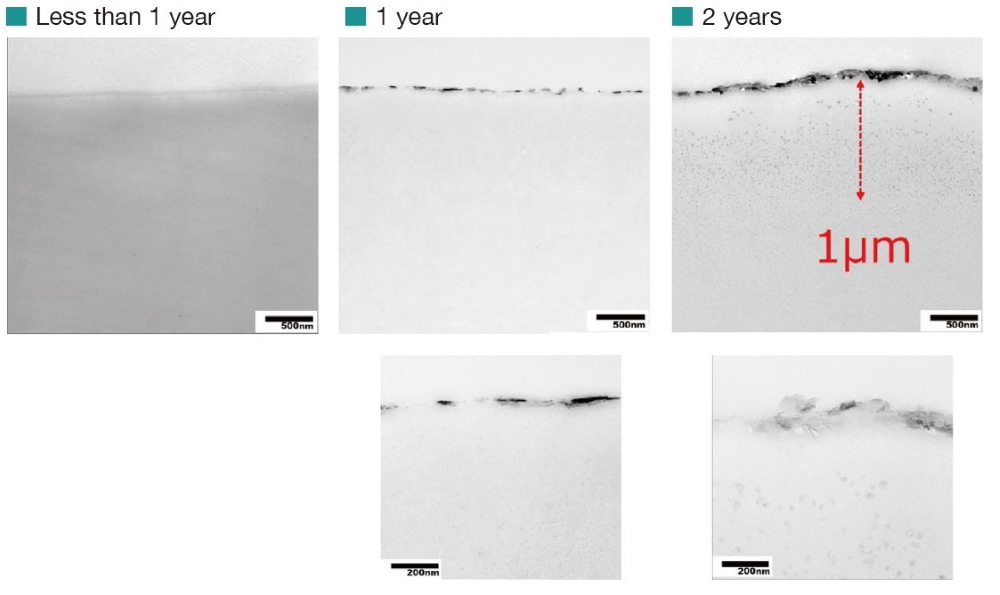
Cross Section Observation by TEM
The cross section near the sample surface was observed using a transmission electron microscope. The result showed that structural changes appeared at a depth of 1 μm on the sample surface with the age of the outdoor exposure testing.
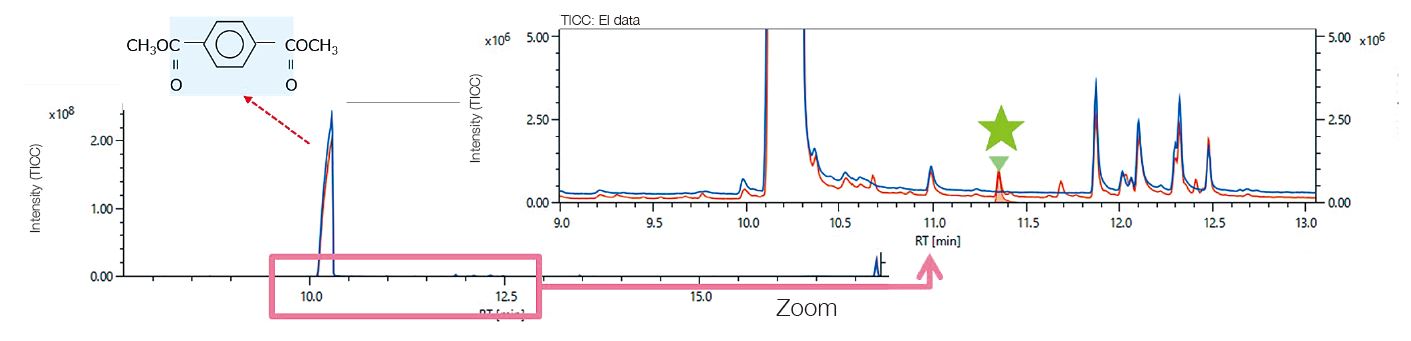
Degradation Analysis by Reactive Pyrolysis GC-MS
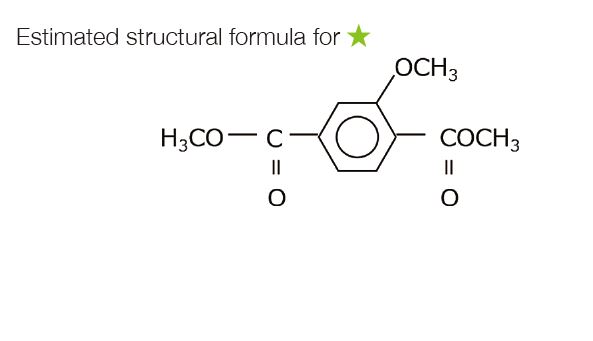
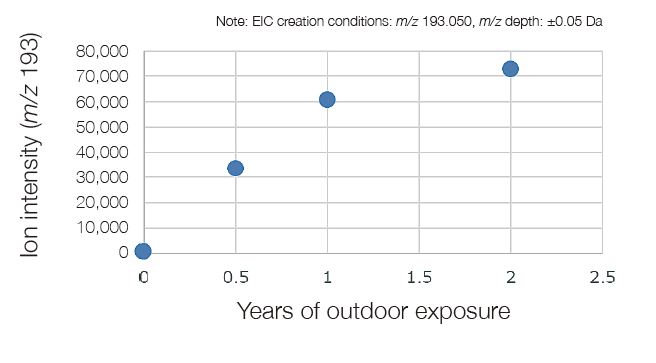
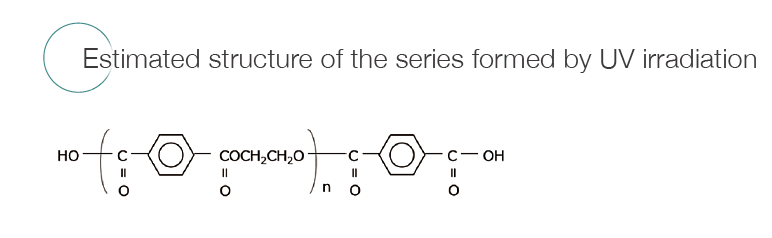
The components, which are reaction pyrolysis products derived from the PET main chain were strongly detected. These were common components before and after UV irradiation. As a result of the difference analysis of the outdoor exposure test between 0 year and 2 years, a component characteristic of the outdoor exposure test was observed at a retention time of 11.37 minutes in the total ion current chromatogram (TICC). The components of the peak that increased from the outdoor exposure test were the same as those which increased due to UV irradiation onto the PET fi lm described on page 36. An EIC was created at the base peak of component ★to confi rm the change of area value with respect to UV irradiation time (average value of n = 3). As a result, it was found that there was a rapid increase within one year of outdoor exposure, and the increase rate became gradual after that.
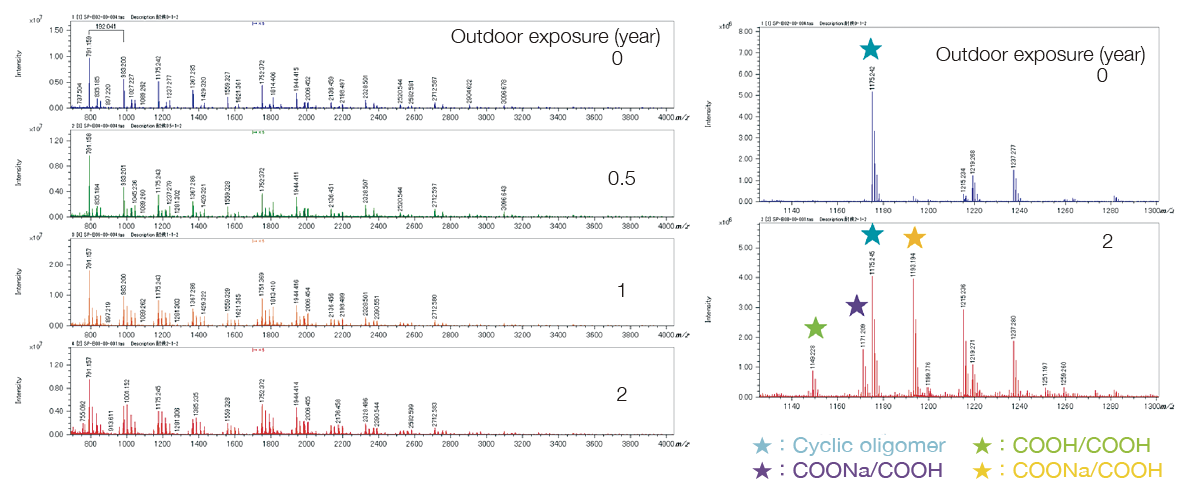
Degradation Analysis by MALDI-TOFMS
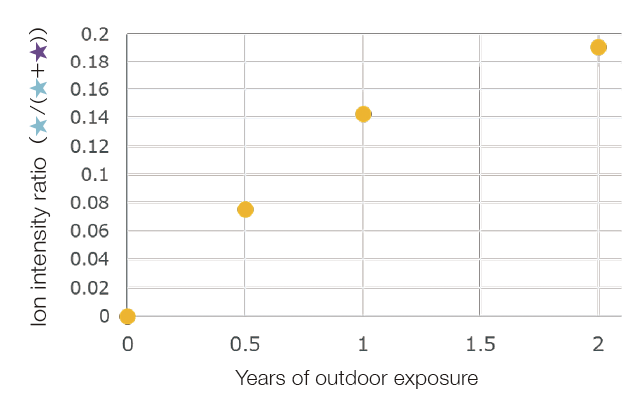
Before the outdoor exposure testing, a series derived from the cyclic oligomer of PET was mainly observed. After the outdoor exposure testing, the series COOX/COOX terminus (X = H, Na), which was presumed to have carboxy terminus derived from the photooxidation reaction, was observed. These were identical to the series observed by UV irradiation onto PET reported on page 36. When the ion intensity ratio of the series with the cyclic oligomer and the carboxy terminal was determined, the carboxy terminal series increased. It was found that the rate of increase during the second year was less than that of the first year.
Characterization of Polymers by JEOL Solutions
Polymernote
JEOL instruments and their applications for polymer analysis are listed in PDF.
Please take a look at this PDF as well.
Surface Analysis & Morphological Observation Instruments

Scanning Electron Microscope (FE-SEM)
The Scanning Electron Microscope (SEM) is an instrument for imaging the specimen surface by irradiating it with a focused electron beam and performing a two-dimensional scan. The SEM detects various signals including secondary electrons, backscattered electrons and characteristic X-rays generated by electron beam irradiation to obtain morphology of the specimen surface, crystalline information and chemical information (composition, etc.). Owing to ease of operation for morphological observation and local area analysis of bulk specimens, the SEM is widely utilized in various applications ranging from basic research to industrial use.

Transmission Electron Microscope (TEM)
The transmission electron microscope (TEM) is an instrument for observing the internal structure of a specimen by passing an electron beam through a thin film specimen. The thickness of the specimen for TEM is less than 100 nm. Generally, since the polymer material is composed of light elements, it is necessary to perform staining with heavy metals. In addition to observing fine structures, TEM can also perform electron diffraction to determine the crystal state of the specimen, elemental analysis, and three-dimensional reconstruction by the tomographic method.
Chemical Analysis Instruments

Mass Spectrometer (GC-MS)
The Gas Chromatograph-Mass Spectrometer (GC-MS) is an instrument for molecular mass measurement of volatile compounds separated by gas chromatography. The Quadrupole Mass Spectrometer (QMS) is the most common mass spectrometer, which is used for various applications due to its compact size and versatility. Recently, the Time-of-Flight Mass Spectrometer (TOFMS) has also gained popularity for its higher resolution. Combined with various pretreatment units, GC-MS can analyze components in gas, liquid and solid samples.

Mass Spectrometer (MALDI-TOFMS)
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometer (MALDI-TOFMS) is a mass spectrometer capable of analyzing substances ranging from low molecular weight compounds such as amino acids to high molecular weight compounds such as synthetic polymers. JMS-S3000 "SpiralTOF™-plus 3.0" is the highest-class resolution MALDI-TOFMS incorporating an ion optical system that has a proprietary spiral trajectory (SpiralTOF ion optical system).
Chemical Analysis Instruments

Nuclear Magnetic Resonance System (NMR)
The Nuclear Magnetic Resonance (NMR) System is an instrument that is used for obtaining data that relates the local chemical environment of a nucleus by irradiating a sample with radio waves while it is placed in a static magnetic field. As each nucleus in a polymer can have a different chemical environment due to factors such as molecular structures and crystalline structures, the observed peak positions (chemical shift values) will vary depending on the surrounding environment. NMR has a large number of applications for both solution and solid samples because it is capable of obtaining a wide range of chemical data including molecular structures, crystalline structures, quantification data and molecular mobility through non-destructive analysis methods.

Electron Spin Resonance (ESR)
ESR is a magnetic resonance instrument that operates on the same principle as NMR. While NMR observes the nuclear spins of the sample and records the spectrum in the absorption waveform, ESR selectively observes only the electron spins of the unpaired electrons in the sample and records them in the first derivative waveform. It is much more sensitive than NMR. Radical reactions are involved in the synthesis process, thermal degradation, and photoaging of polymers. Many of the reactions of polymerization inhibitors, antioxidants, and light absorbers used to control them are also considered to be radical-mediated. These reactions can be observed using ESR.
Surface Analysis & Morphological Observation Instruments

X-ray Photoelectron Spectrometer(XPS)
The X-ray Photoelectron Spectrometer (XPS) is an analysis instrument capable of quantitative, qualitative, and chemical-bonding state analyses of the top surface of a substance by analyzing photoelectrons that are generated when a specimen is irradiated with X-rays. Other applications of XPS include specimen surface cleaning by ion irradiation and internal structure analysis by repeated measurement and etching.
Surface Analysis & Morphological Observation Instruments

X-ray Fluorescence Spectrometer (XRF)
The X-ray Fluorescence Spectrometer (XRF) enables analysis of constituent elements and compositions by detecting fluorescent X-rays emitted from a specimen irradiated with X-rays. For polymer materials, XRF is a quick and reliable method for quality control analysis in connection with product safety. It can be used for quick screening of residual catalyst used for polymerization, RoHS-restricted heavy metal elements (Cd, Pb, Hg, Cr and Br) as well as analysis of inorganic additive content used for functional improvement. Possible applications of the spectrometer also include analyzing inorganic surface treatment film deposits and thickness and identifying contamination mixed in during the manufacturing process. XRF is an element analysis instrument capable of easily analyzing elements that constitute a material in a non-destructive manner.
To see more Plastics/Polymer applications, click the button.

