Medicine / Drug discovery
State-of-the-art scientific instruments are indispensable for medicine and drug discovery research technologies. JEOL offers structural analysis solutions for medicine and drug discovery by linking technologies,including electron microscopes, nuclear magnetic resonance spectrometers, and mass spectrometers. This page introduces analysis methods and application examples ranging from crystallograpphy workflow to single particle structure analysis using the most advanced cryo electron microscopes.
Structural analysis by Cryo-TEM
Cryo-electron microscopes are specialized in the observation of electron beam-sensitive specimens, such as proteins.
It is applicable for methods such as single particle structure analysis, tomography, and electron crystallography.
GroEL
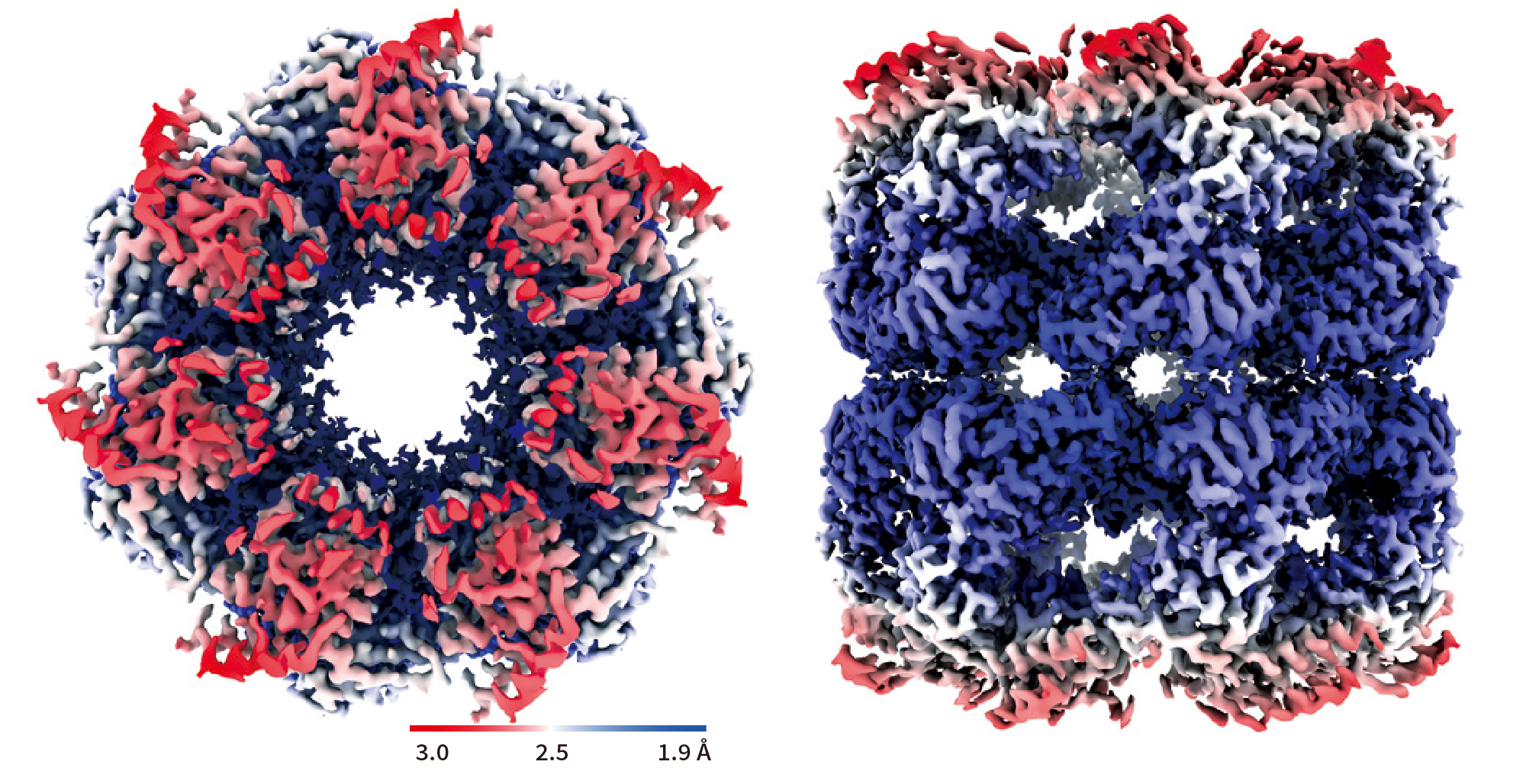
- GroEL structure at 1.98 Å resolution achieved by only 504 micrographs
- In a previous study,GroEL structure at 3.1 Å resolution was obtained from 1,833 micrographs.(as of Oct. 26, 2020 at EMDB)
Data:courtesy of Dr. Junso Fujita at Osaka University
Hemoglobin
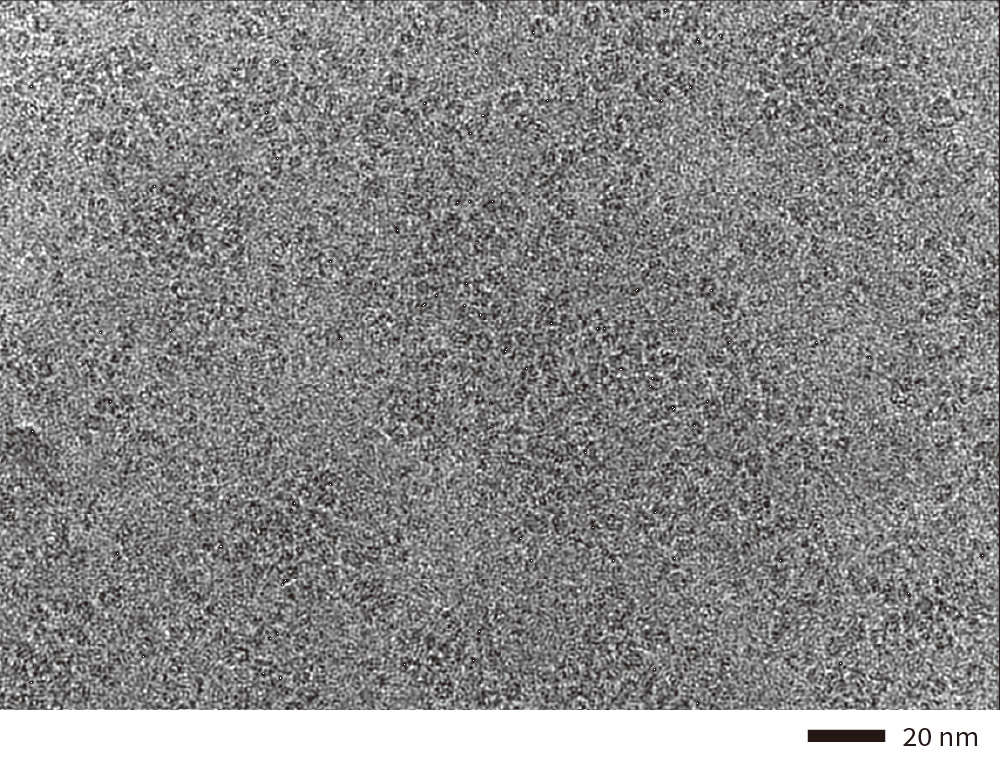
Cryo-electron micrograph(left), 3D density map(center), and fitted atomic model(right) of human hemoglobin obtained by high speed data collection, 850 micrographs per hour.
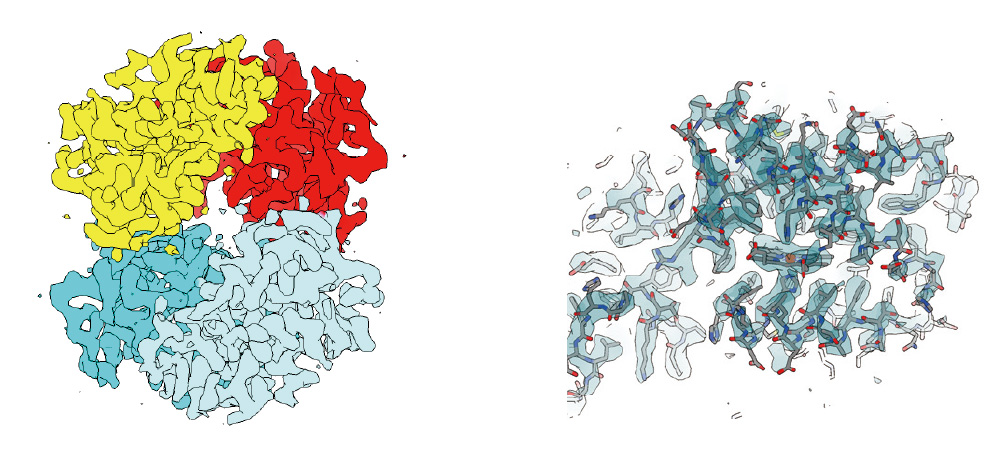
Data:courtesy of Dr. Miki Kinoshita at Osaka University
Exosome

- Cryo-electron tomogram of isolated exosomes with a hole-free phase plate.
- The 3D reconstructured image(tomogram)was obtained from the micrographs of a tilt range from -60°to +60° with steps of 2°.
Sample:courtesy of Dr. Naoomi Tominaga at National Cancer Center Reseach Instrumente and The University of Tokyo.
Innexin-6 gap junction channel
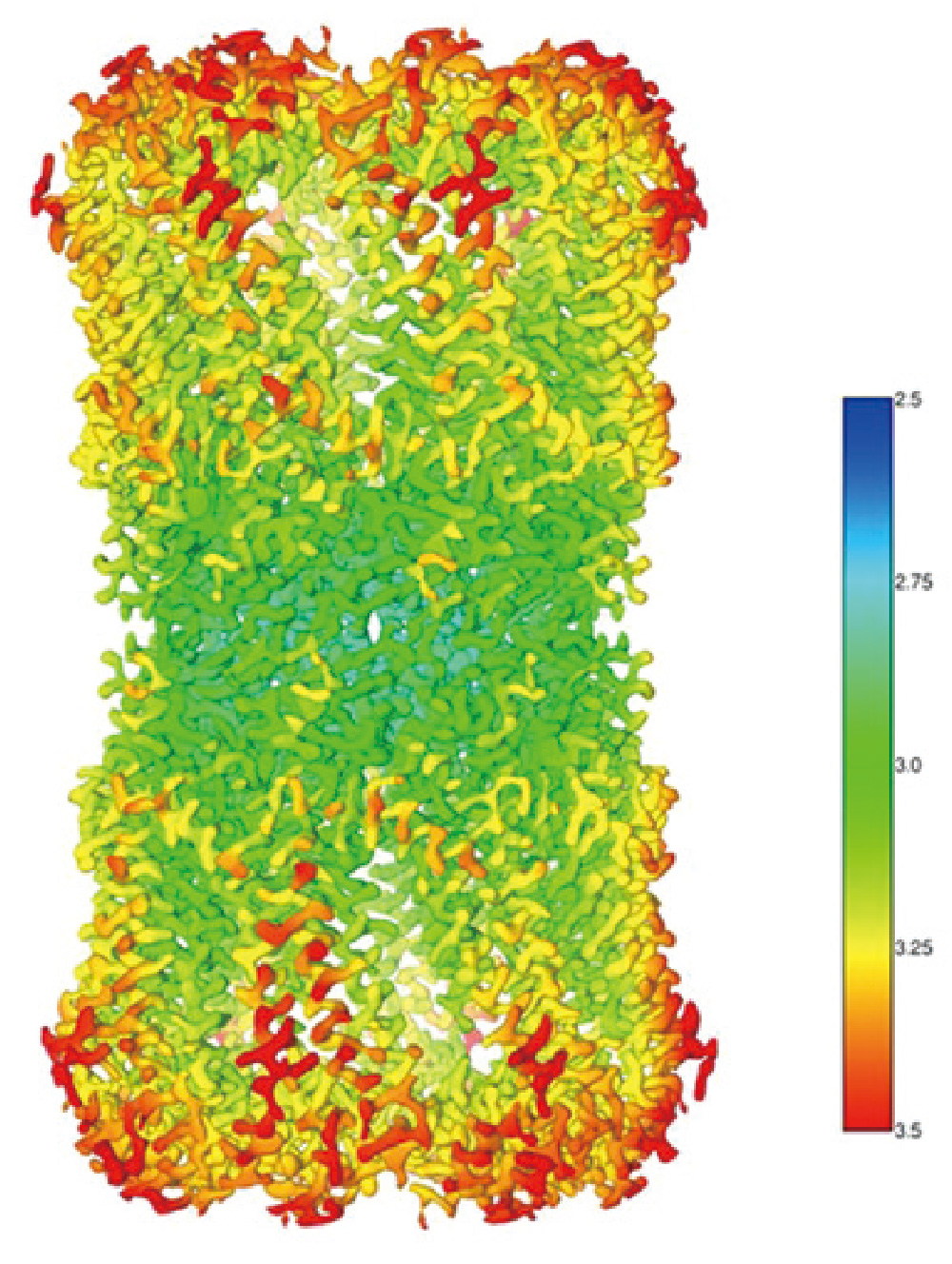
- Sample: Innexin-6 (Caenorhabditis elegans)
- Microscope: CRYO ARM™ 300 (300 kV CFEG) with Gatan K2
- Software used for image acquisition : JADAS (1974 images)
- Number of particles: 91,613 (Initial pickup), 37,767 (for 3D reconstruction)
- Software used for image analysis: Relion3
- Resolution: 3.0 Å (at FSC = 0.143)
Sample by courtesy of Prof. A. Oshima (Nagoya University)
Apoferritin
The highest resolution 1.53 Å achieved by cryoEM 2019.02
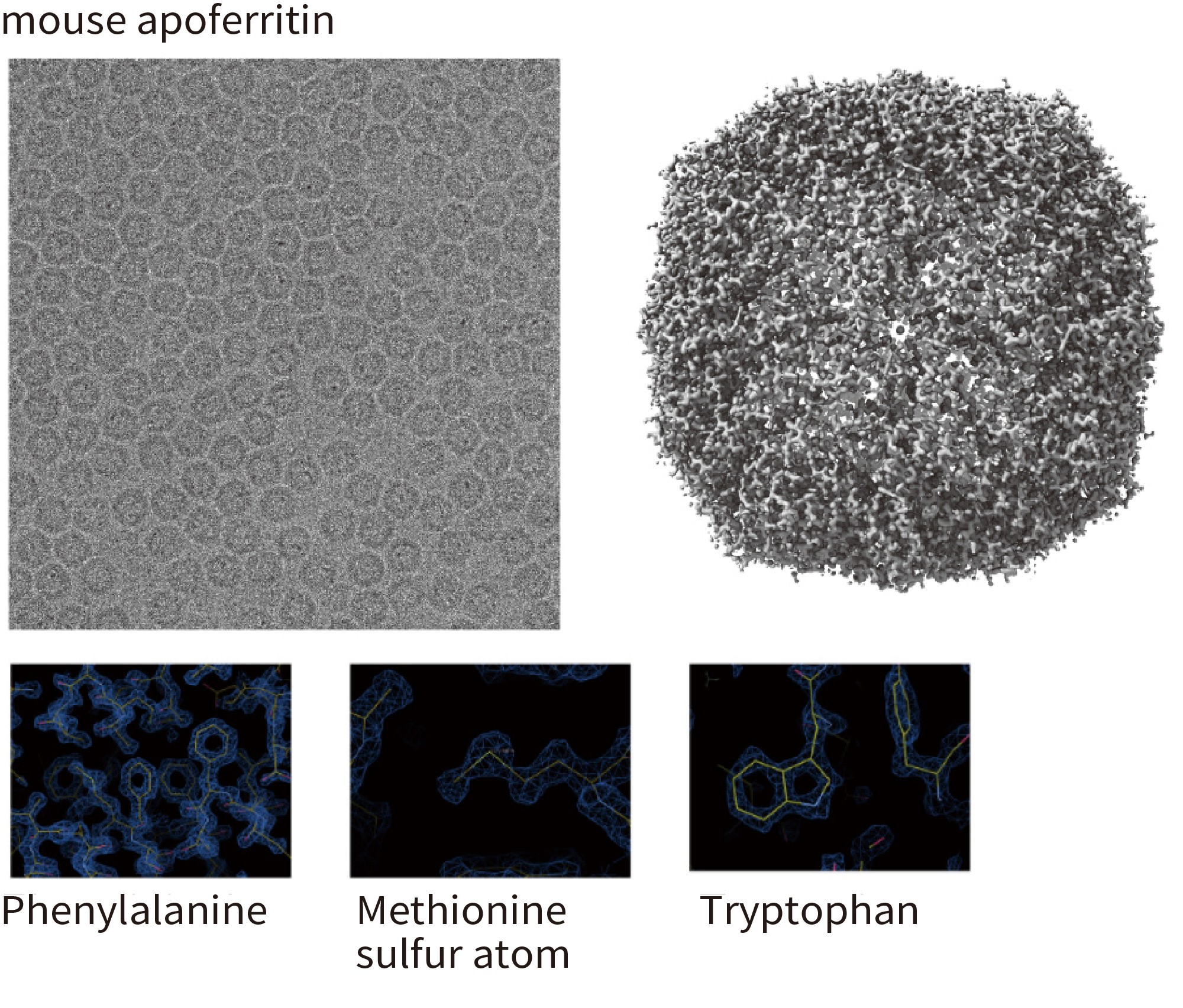
- Optics features: Cold FEG 300 kV & Ω -type energy fi lter with 20 eV slit width
- Detector: Gatan K2 (image pixel size: 0.495 Å, mag x 100,000)
- Grid: Quantifoil 1.2/1.3 Cu 200 mesh, kept in the autoloader for 3 days before data collection
- No. of micrographs: 974 collected over 24 h, 840 used for image analysis
- No. of particles: 120,295 used for fi nal reconstruction
- Software: RELION 3.1b, CTFFIND4
- Resolution: 1.53 Å (B-factor: 47)
- Note: the first 56 images alone produced a map of 1.76 Å resolution (B-factor: 45)
Mouse apoferritin plasmid from Yanagisawa, Danev & Kikkawa @Tokyo University Kato, Makino, Nakane, Terahara, Kaneko, Shimizu, Motoki, Ishikawa, Yonekura & Namba 2019.02 (EMDB-9865)
B -galactosidase
β -galactosidase 2.43 Å resolution CRYO ARM™
3D image of ß-galactosidase using cryo electron microscope (CRYO ARM™ 200) at 200 kV accelerating voltage.
The figure shows the structure ofβ
-galactosidase revealed by a research team led by Specially Appointed Professor Keiichi Namba and Assistant Professor Takayuki Kato of the Graduate School of Frontier Biosciences, Osaka University, using a cryo-EM,CRYOARM™ equipped with a Schottky-type FEG at an accelerating voltage of 200 kV.
This data was verified by the Fourie Shell Correlation method (graph), resulting in excellent resolution of 2.43 Å in a 3D reconstructed image.
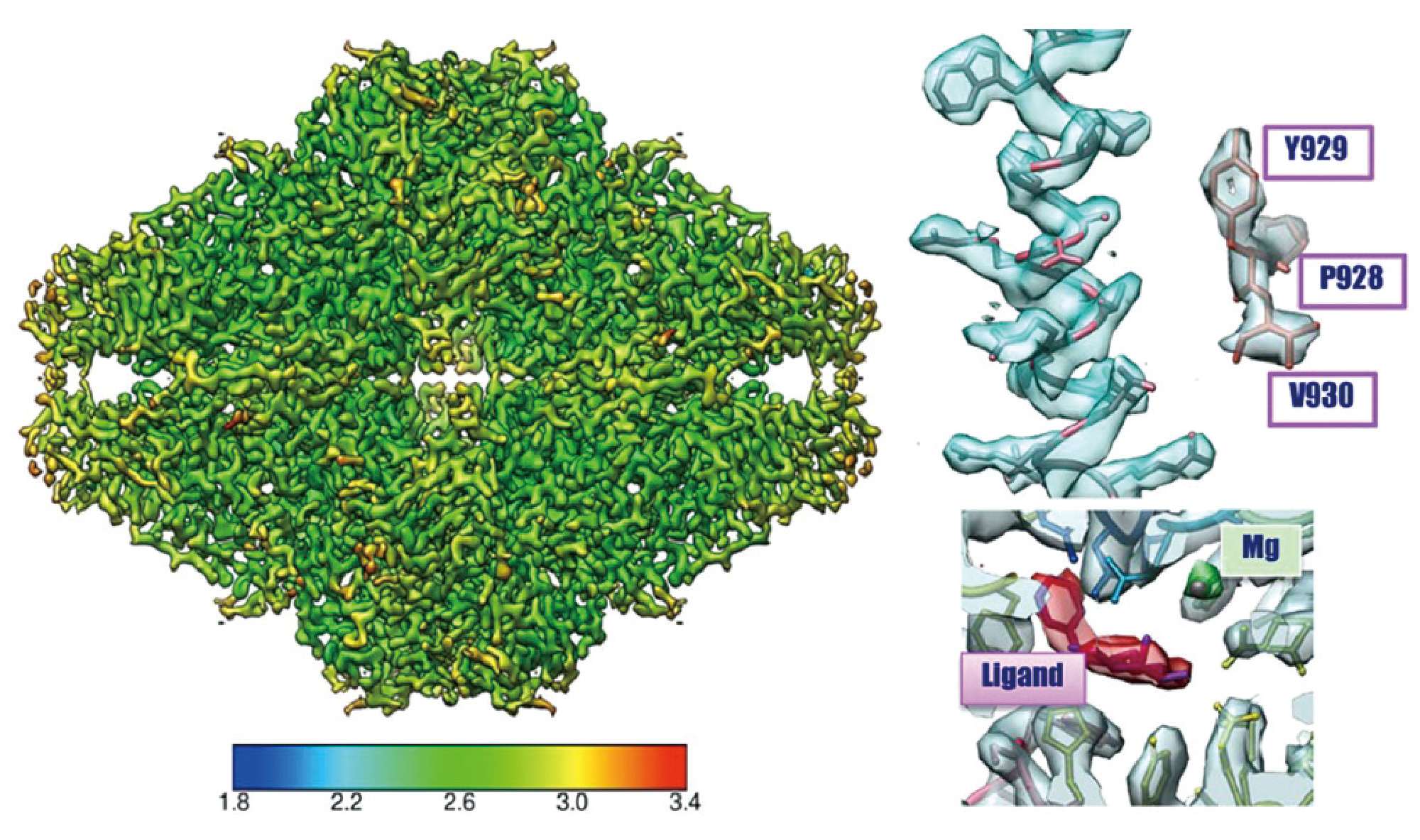
- Sample: β -galactosidase with PETG
- Microscope: CRYO ARM™ (Schottky 200 kV) / K2 summit
- Grid: Quantifoil 1.2/1.3 Cu 200 mesh, kept in the autoloader for 3 days before data collection
- Number of Images: 2,500 over 3 days by JADAS
- Image pixel size: 0.8 Å/pixel
- Number of particle images: 350,000 (Initial pickup), 88,564 (for final 3D reconstruction)
- Software: Motioncor2, Gctf, Gautomatch, Relion2.0
- Total dose: 70 e-/Å2 (70 frames (0.2 sec/frames x 14 sec)
Data: courtesy by Dr. T. Kato and Dr. K. Namba, Osaka University, August 2017

JEM-3300 CRYO ARM™ 300 II Field Emission Cryo-Electron Microscope
The JEM-3300 "CRYO ARM™ 300 II" is a cryo-electron microscope that specializes in the observation of electron beam-sensitive specimens, such as proteins, for single particle analysis, tomography and MicroED. This system offers improved stability, throughput and ease of use compared to the previous generation of cryo-EMs. Moreover, this is an all-in-one system that can handle everything from screening to data acquisition, allowing for more flexibility in operation at customer sites to meet the needs of the facility. These improvements allow users to obtain high quality images by simple operation even for those who have never used an electron microscope before.
Introduction of Workflow for Analysis of Organic Compounds
Characteristics of electron diffraction structure analysis using XtaLAB Synergy-ED
Electron diffraction structure analysis is one of the methods to determine the three-dimensional structure of a molecule. It is possible to unambiguously determine the three-dimensional configuration of all atoms in complex molecules without the need for the crystallization process required in general single crystal X-ray structure analysis.
Structure refinement based on MS and NMR analysis results
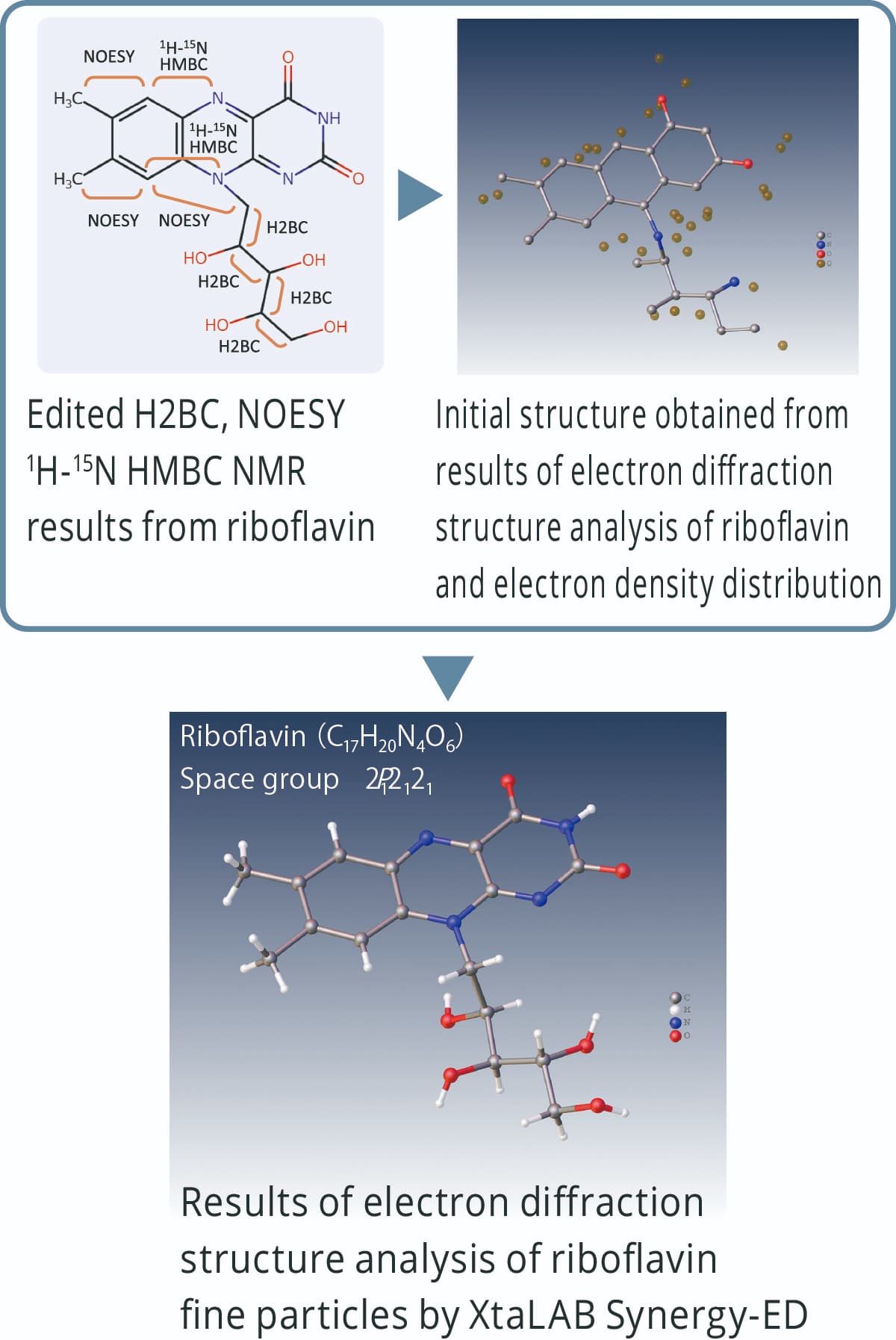
Electron diffraction structure analysis is performed based on the electron density distribution. In the case of molecules composed of elements with similar atomic numbers, it may be difficult to estimate the difference between each element and the number of hydrogen atoms. Initial structures derived from electron diffraction analysis can be refined using chemical structure information obtained from MS and NMR.
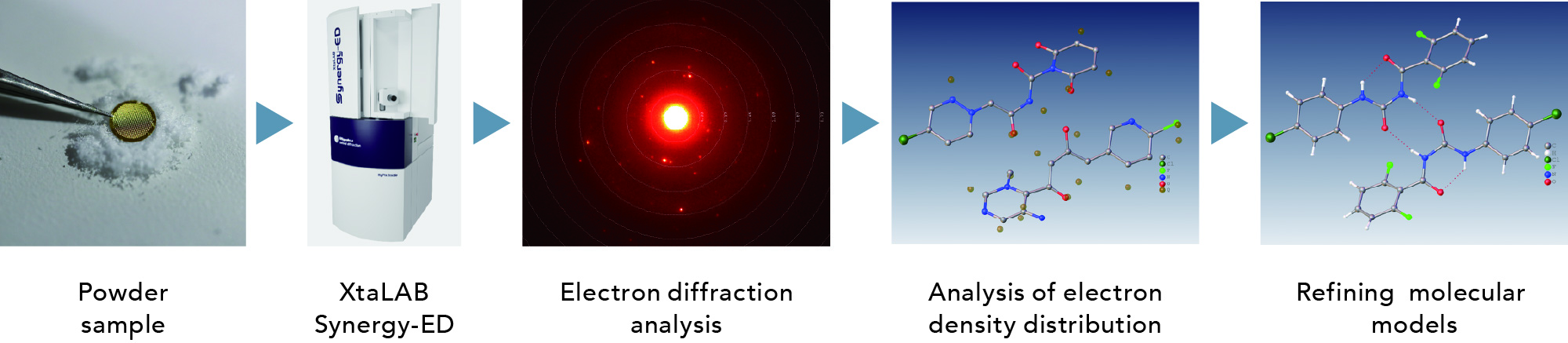
Organic compound analysis workflow
The XtaLAB Synergy-ED can perform electron diffraction structure analysis on powder samples such as test reagents in their original state. In addition, it can be combined with JEOL's mass spectrometer (MS) and nuclear magnetic resonance spectrometer (NMR) for precise chemical structure analysis of sub-micron crystals.
Analysis example of alkaloid organic compounds
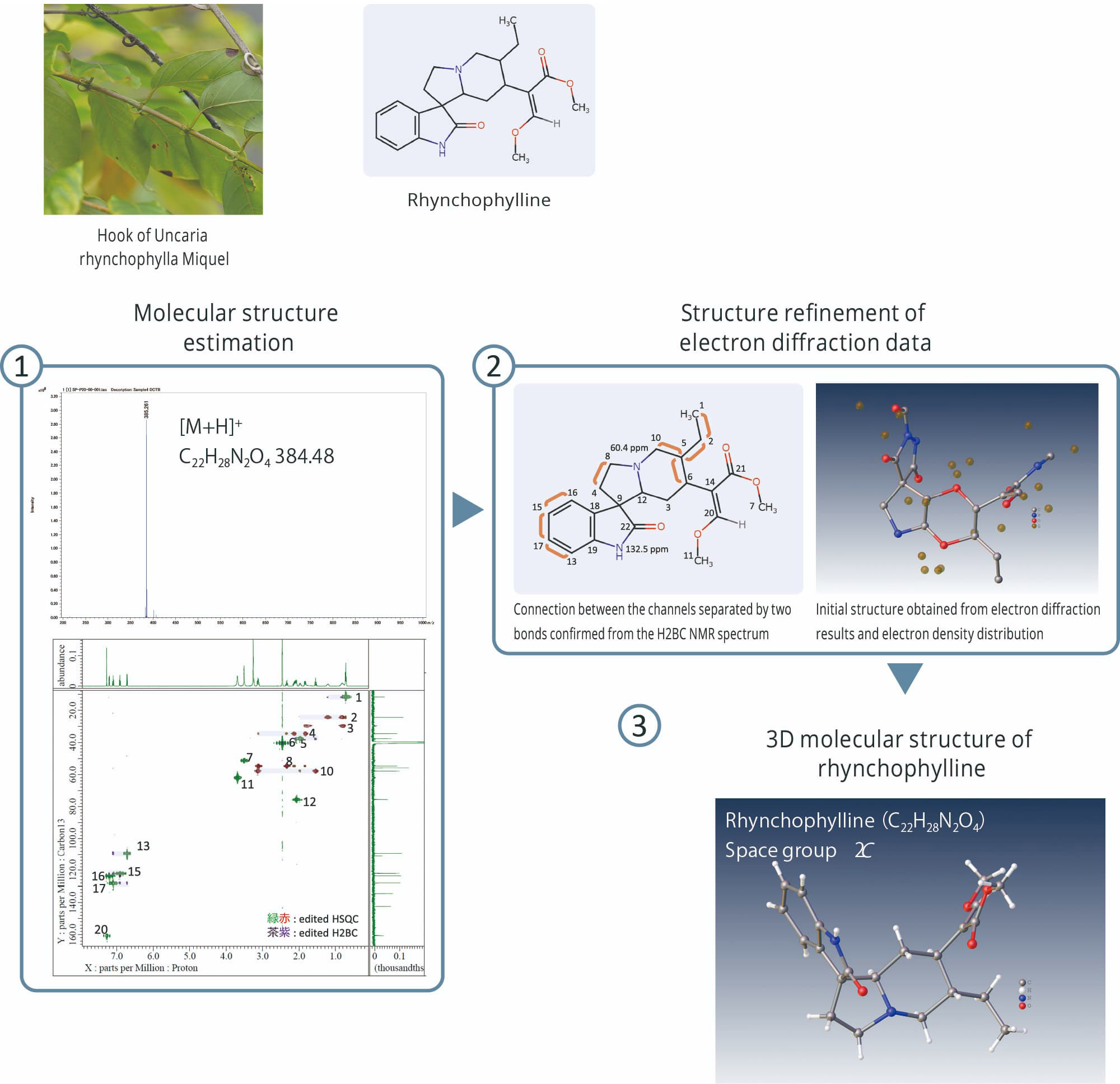
① Top: Accurate mass spectrometry of rhynchophylline by JMS-S3000 SpiralTOF™-plus 3.0
① Bottom: Edited HSQC and edited H2BC NMR spectra of Rhynchophylline by JNM-ECZL 500R

① Edited HSQC and edited H2BC NMR spectra of Cinchonidine by JNM-ECZL 500R
② Connection between the channels separated by two bonds confirmed from the edited H2BC NMR spectrum of
cinchonidine by JNM-ECZL 500R
③ Result of electron diffraction structure analysis of cinchonidine fine particles by XtaLAB Synergy-ED
Microcrystalline Structure Analysis of Gefitinib
Determination of crystal structure
Accurate structure analysis using The XtaLAB Synergy-ED and JEOL's mass spectrometers and nuclear magnetic resonance equipment enables more accurate crystal structure determination.

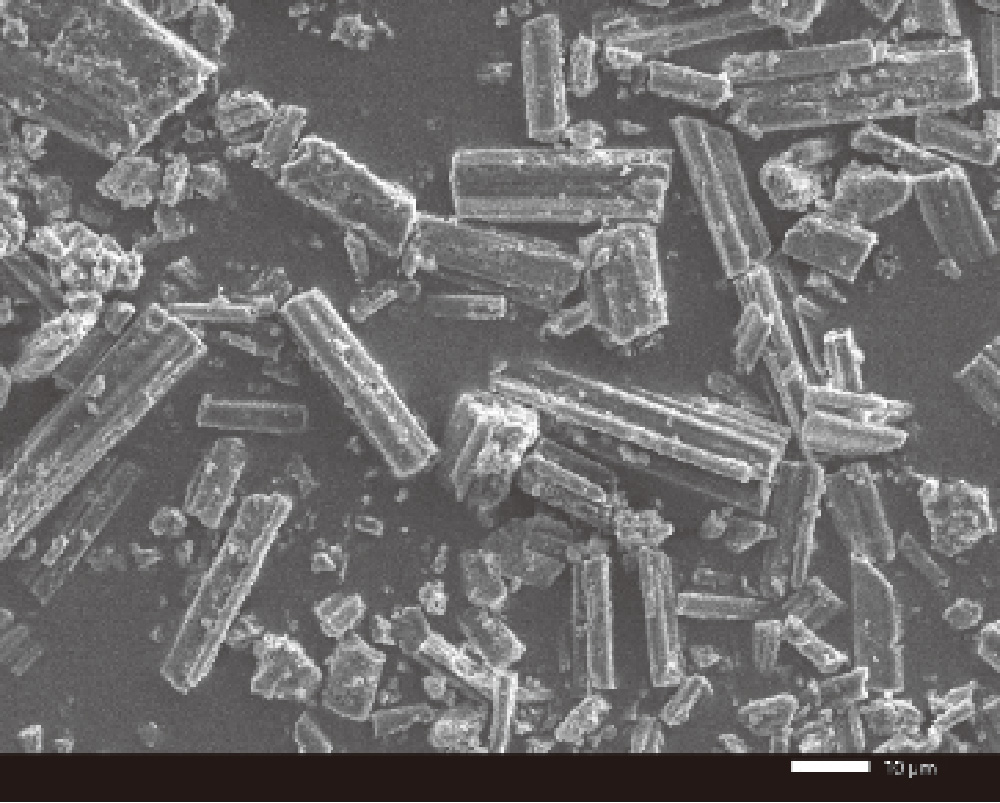
JSM-IT200 7 kV × 1,000 secondary electron image
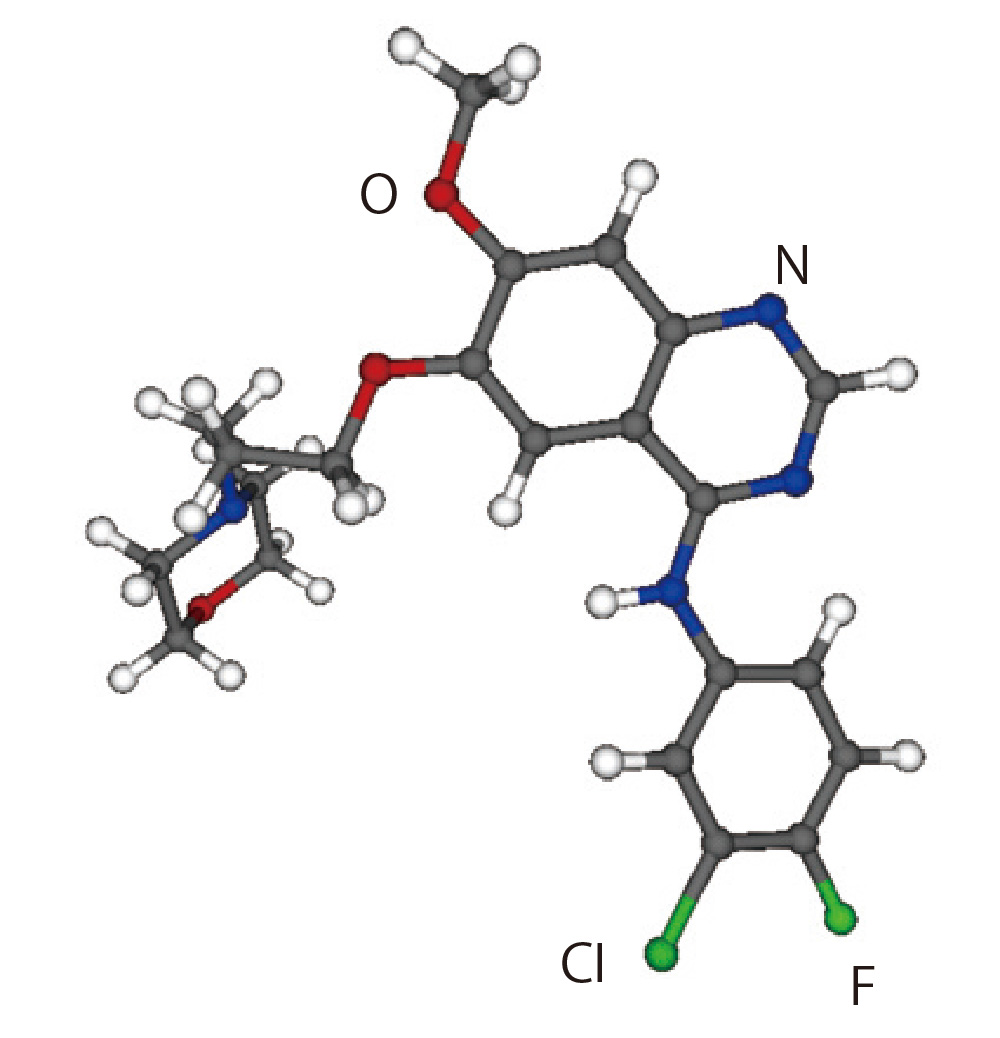
Gefitinib : C22H24N4O3FCl
Crystal Structure Analysis Workflow

Microcrystal structure analysis by XtaLAB Synergy-ED
It is possible to analyze the crystal structure of gefitinib in the state of
microcrystals.
Two estimated structures were obtained from the sample.


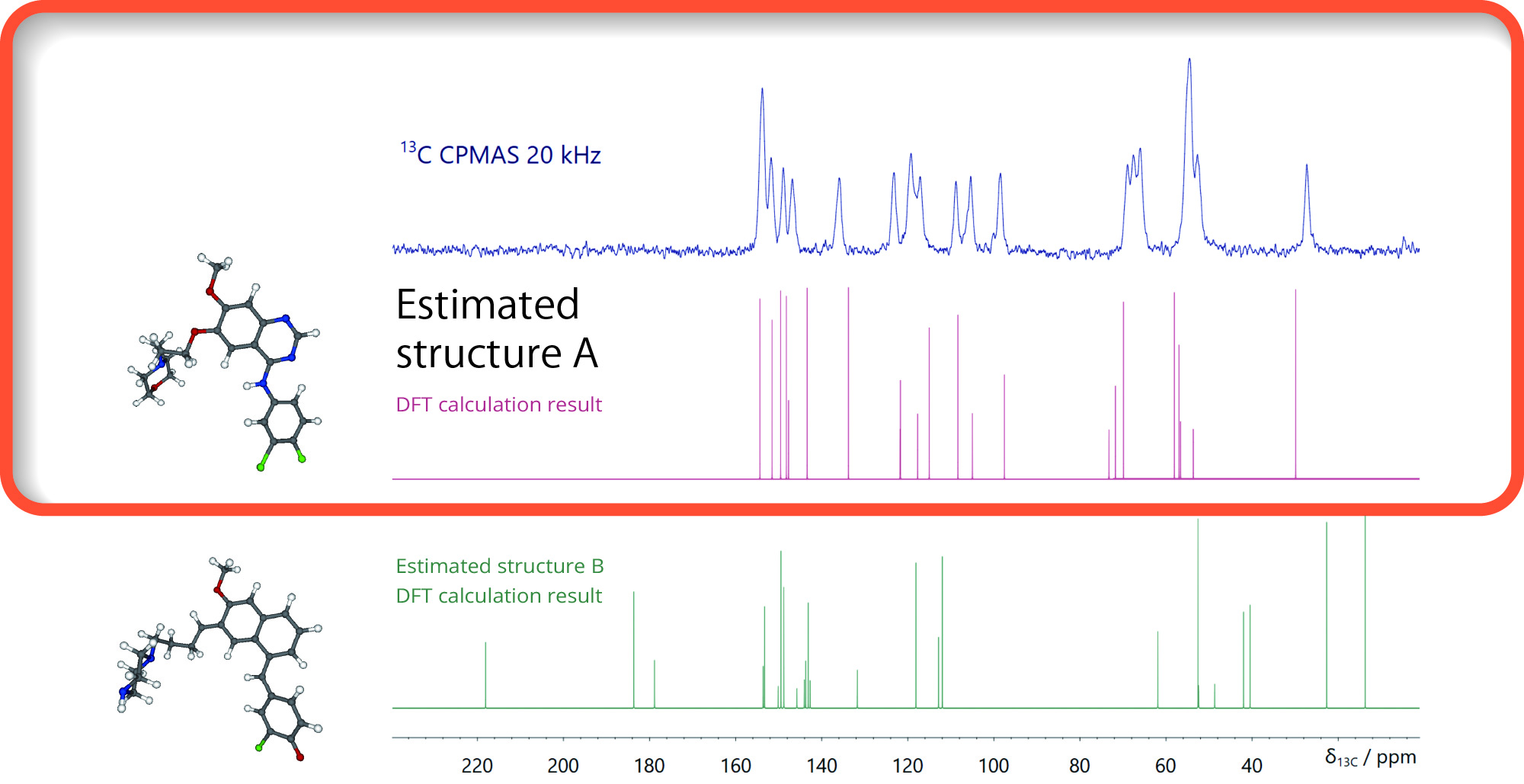
NMR crystal structure analysis by solid-state JNM-ECZL600 NMR
The crystal structure can be analyzed by performing DFT calculations with the estimated structure as the initial structure and comparing it with the solid-state NMR 13C chemical shift. Here, the possibility of the estimated structure A was shown.

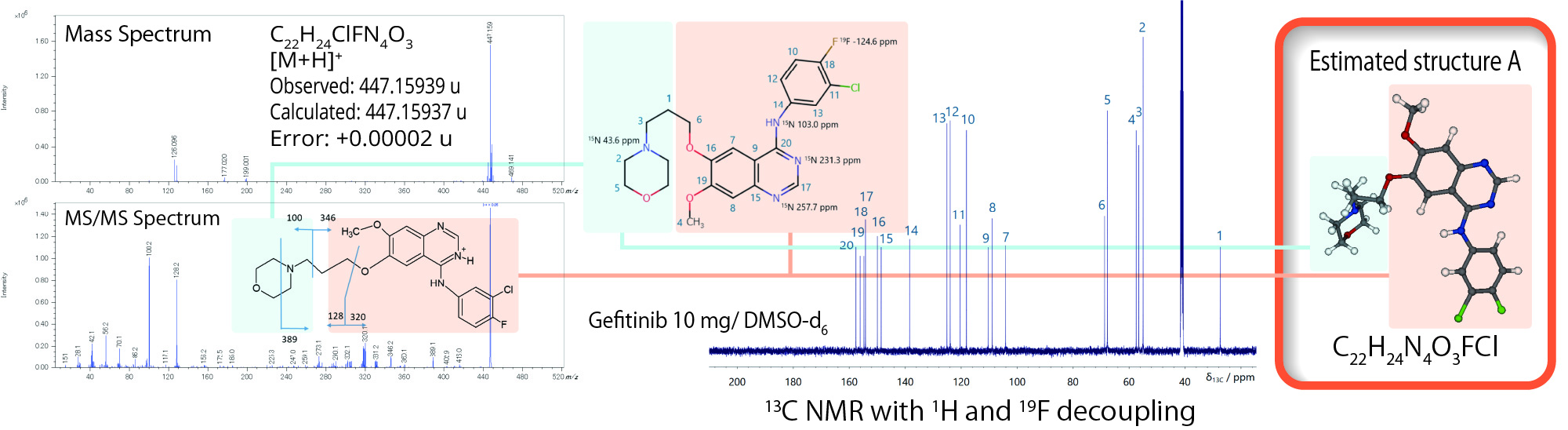
Molecular structure analysis by JMS-S3000 SpiralTOF™-plus 3.0 and solution NMR
The results of the molecular structure analysis by mass spectrometry and solution NMR agreed with the estimated structure A.

XtaLAB Synergy-ED Electron Diffractometer
The XtaLAB Synergy-ED is a new and fully integrated electron diffractometer that uses electron beams to create a seamless workflow from data collection to structure determination of three-dimensional molecular structures. The XtaLAB Synergy-ED is the result of combining core technologies to achieve synergy between Rigaku's high-speed, high-sensitivity detector (HyPix-ED) and single crystal analysis software (CrysAlisPro for ED) and JEOL's transmission electron microscope technology. By creating a seamless workflow from sample selection (nanocrystals) to data collection and structure analysis, the XtaLAB Synergy-ED makes electron diffraction easily accessible to non-experts who do not have the expertise in electron microscopy and crystallography that is typically required.

JNM-ECZL (ECZ Luminous™) series FT NMR System
The JNM-ECZL series (ECZ Luminous™) is an FT NMR system equipped with state-of-the-art digital and high-frequency technologies. The highly integrated Smart Transceiver System, a high-speed, high-precision digital high-frequency control circuit, enables further miniaturization and ensures the stability of spectrometer. The new Multi Frequency Drive System enables multi-resonance measurements in a standard configuration, providing a wider range of solutions.

JMS-S3000 SpiralTOF™-plus 3.0 Ultra-High Mass-Resolution MALDI-TOFMS System
The JMS-S3000 SpiralTOF™-plus 3.0 is a MALDI-TOFMS* that uses innovative SpiralTOF ion optics that has been updated to extend the mass range of this high-resolution system. The JMS-S3000 defines a new standard in MALDI-TOFMS performance and provides state-of-the-art analytical solutions for a wide range of research areas such as functional synthetic polymers, materials science, and biomolecules.
* Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometer

JMS-T2000GC AccuTOF™ GC-Alpha High Performance Gas Chromatograph - Time-of-Flight Mass Spectrometer
The JMS-T2000GC AccuTOF™ GC-Alpha is a high-performance GC-MS system that simultaneously realizes high mass resolution, high mass accuracy, high sensitivity, high speed data acquisition, wide dynamic range, and wide mass range. Two types of multi-ionization combination ion sources - EI/FI/FD combination ion source and EI/PI combination ion source - are available. Soft ionization methods can produce molecular ions from unknown compounds whose molecular ions can not be produced by EI, and facilitate the elucidation of their elemental compositions. The system comes with the automatic structure analysis software "msFineAnalysis AI" for integrated structure analysis of the data measured by the multiionization combination ion sources, thus providing fast automatic structure analysis, which does not solely depend on mass spectral library databases.
Chemical Analysis Equipment
JEOL instruments utilized in the medicine and drug discovery fields and their applications are contained in a PDF.
Please take a look at this as well.
To view applications on Medicine / Drug discovery more, click button below.
Contacts
JEOL provides a variety of support services to ensure that our customers can use our products with peace of mind.
Please feel free to contact us.

