Is Quantitative NMR Changing Quantitative Analysis (2)?
Issue 7
For the 7th issue we continue the discussion of “Is qNMR changing quantitative analysis?”
How can we solve the carminic acid problem?
Solution hint
The “carminic acid problem” here is the possibility that the analysis results will be different depending on the standard material that is used. If there was a way to know the absolute purity of each standard material, the problem could be solved by applying a calibration to the results. The absolute purity of an organic compound is determined by a method called a Primary Standard Measurement Method, which includes methods like freezing point depression and titration. Although these methods enable highly-accurate analysis, they are not general-purpose methods, because there are cases where an assessment is not possible due to the nature of the substance or operability issues. In contrast, as discussed previously (Issue 4), NMR can be used to determine the purity or concentration of an analyte by using any reference material with a known purity or concentration. Furthermore, if the appropriate procedures are followed, it is possible to make SI-traceable assessments of purity (Issue 5). This is why the use of NMR is becoming so widespread. Purity assessment of an organic substance using quantitative NMR can be regarded as a suitable method for assessing the purity of standard material and reference materials.
New approach for quantitative analysis
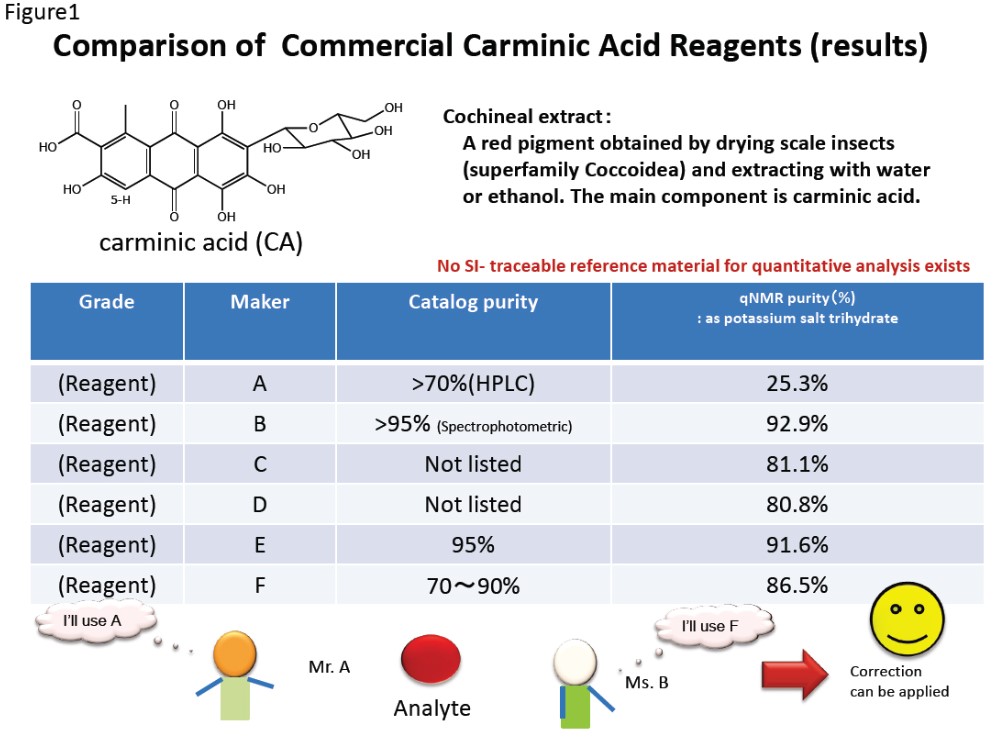
The results obtained using quantitative NMR to assess the purity of various carminic acid samples are shown here.
Figure 1 it is clear that the purity could be determined for all the carminic acid reagents. With this information, it is possible to apply a calibration, no matter which carminc acid reagent is used as the standard substance when performing a chromatographic analysis, making it possible to obtain the same results for the same sample, no matter who performs the measurement. This allows us to propose a new approach for quantitative analysis.
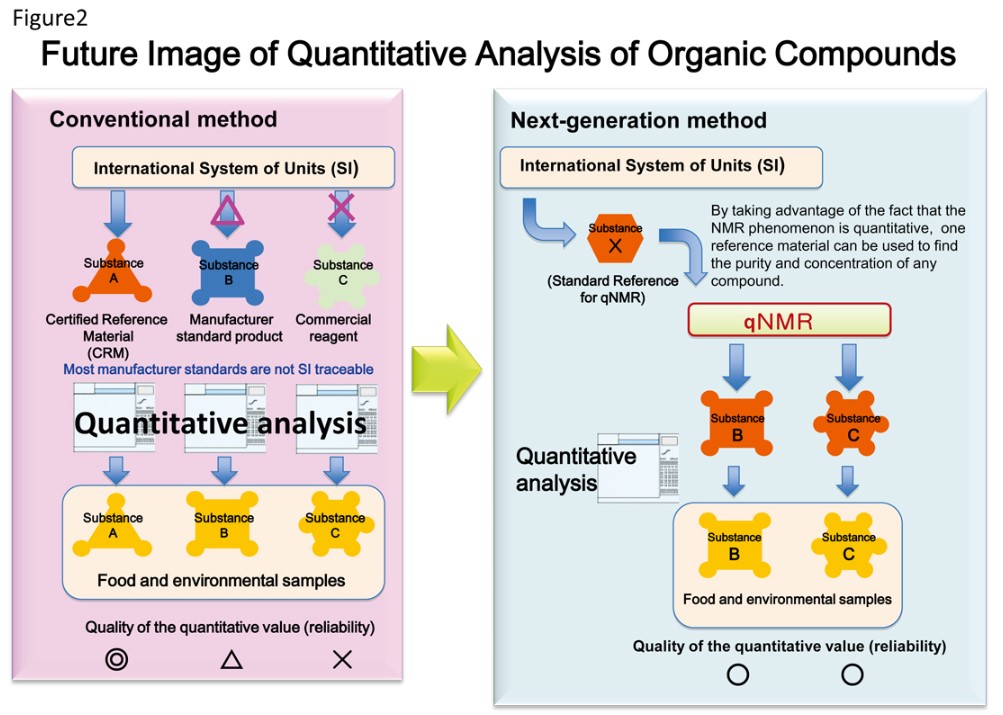
Figure 2 shows an outline of a system in which the quantitative results do not depend on the quality of the reference material. This concept is started to be adopted in compendial methods, and is expected to become the approach of quantitative analysis in the near future.
What the carminic acid example teaches us
The contribution of quantitative NMR as a method of solving the problem of the standard materials, which significantly affect the results of chromatographic analysis, has been introduced. Although it can be used to improve the reliability of quantitative analysis results, there may be an even deeper significance to being able to know the actual purity of organic compounds. This is because knowing the purity of an organic compound can influence the assessment and processes in later stages. For example, a substance with a pharmacological activity or toxicity. Isn’t there some relationship between the purity and the assessment of pharmacological activity or toxicity? Or something that is used as a raw material.
There is likely to be a relationship with reaction efficiencies in later processes. From this perspective, it is easy to imagine how knowing the purity of an organic compound may have a wide range of applications. And, if it can be accomplished with a fairly familiar tool like NMR…the applications for quantitative NMR will surely continue to expand.
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
