The reason quantitative analysis is possible with NMR
Issue 3
Why is quantification possible?
The area (integrated value) of a signal on the 1H spectrum is proportional to the molar concentration and number of protons of the substance corresponding to the signal.
I = kcH
(I is the integrated area of the signal, k is a constant, c is the molar concentration and H
is the number of protons)
With NMR, the properties of the molecules (like UV, absorbance, etc.) are not observed the way they are in a chromatographic method. Therefore, this relationship remains the same even if the molecules are different. In other words, the response factor does not depend on the molecule. This is a key feature of quantitative analysis with NMR.
2 molecules are used to present a somewhat more specific example of how the calculations are made.
Figure 1 shows the 1H spectrum of a solution containing acetaminophen and methyl hydroxybenzoate at the same molar concentration.
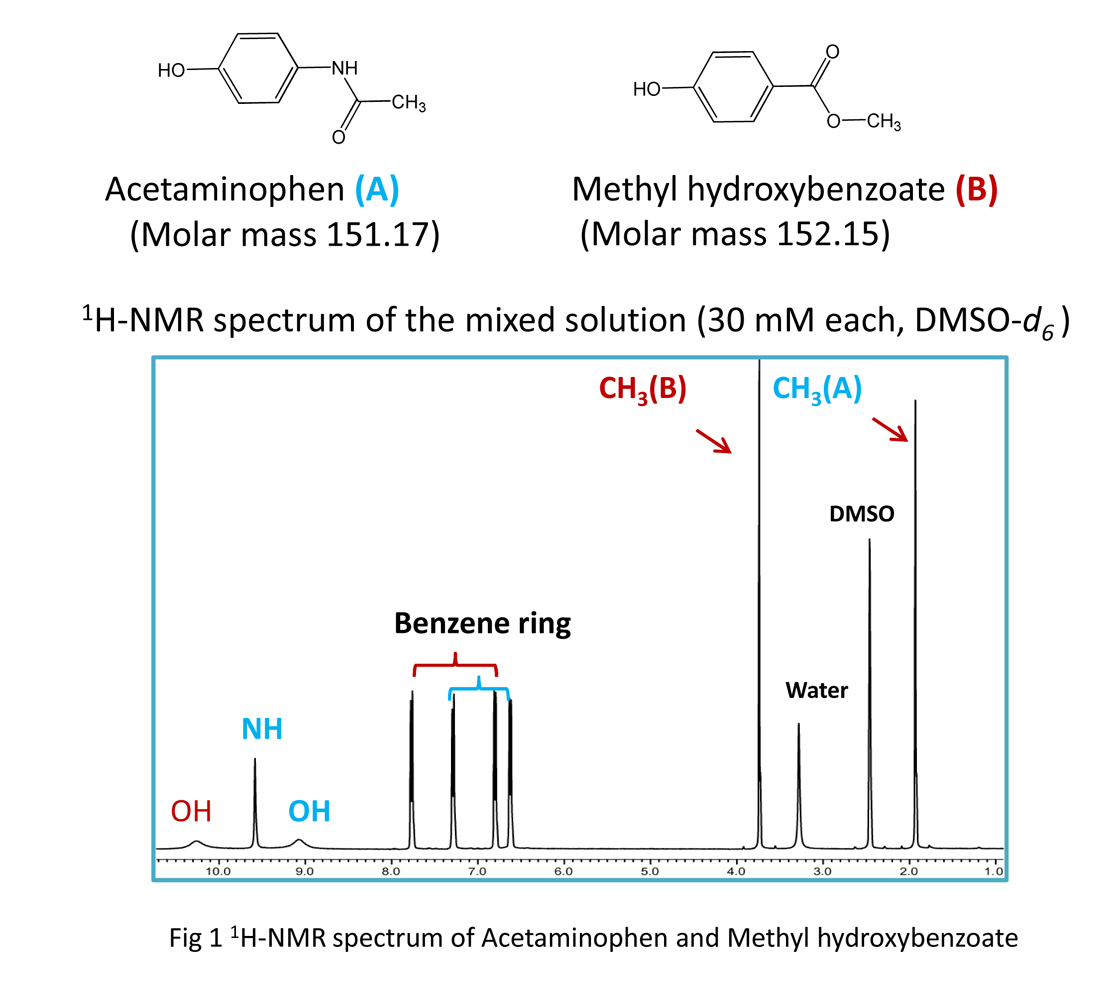
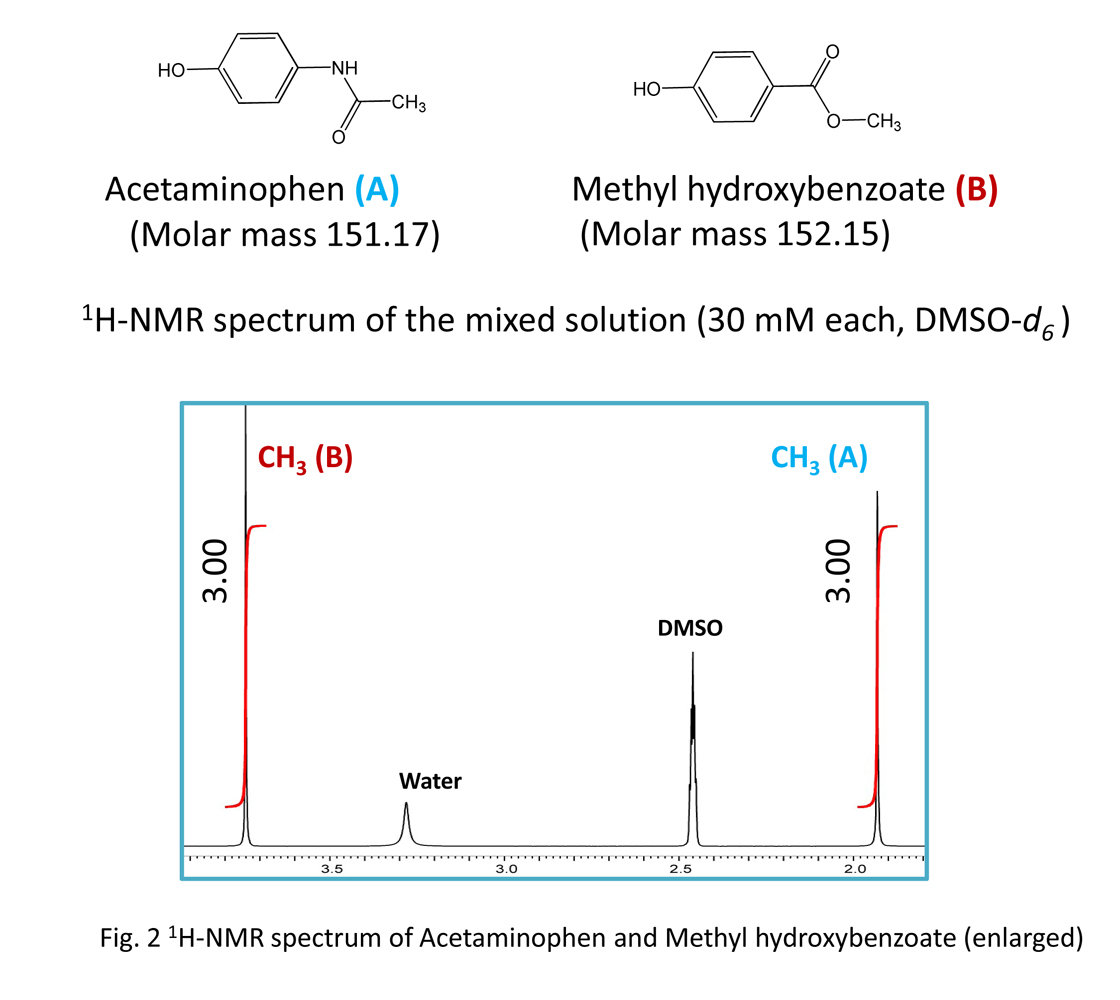
Although the structure of these 2 molecules is similar, the spectral patterns are different. If we focus on the methyl group in these 2 molecules (Fig. 2), since the molar concentration is to be prepared the same, we see the same integrated value, even though the molecules are different. This clearly shows that the response factor does not depend on the molecule.
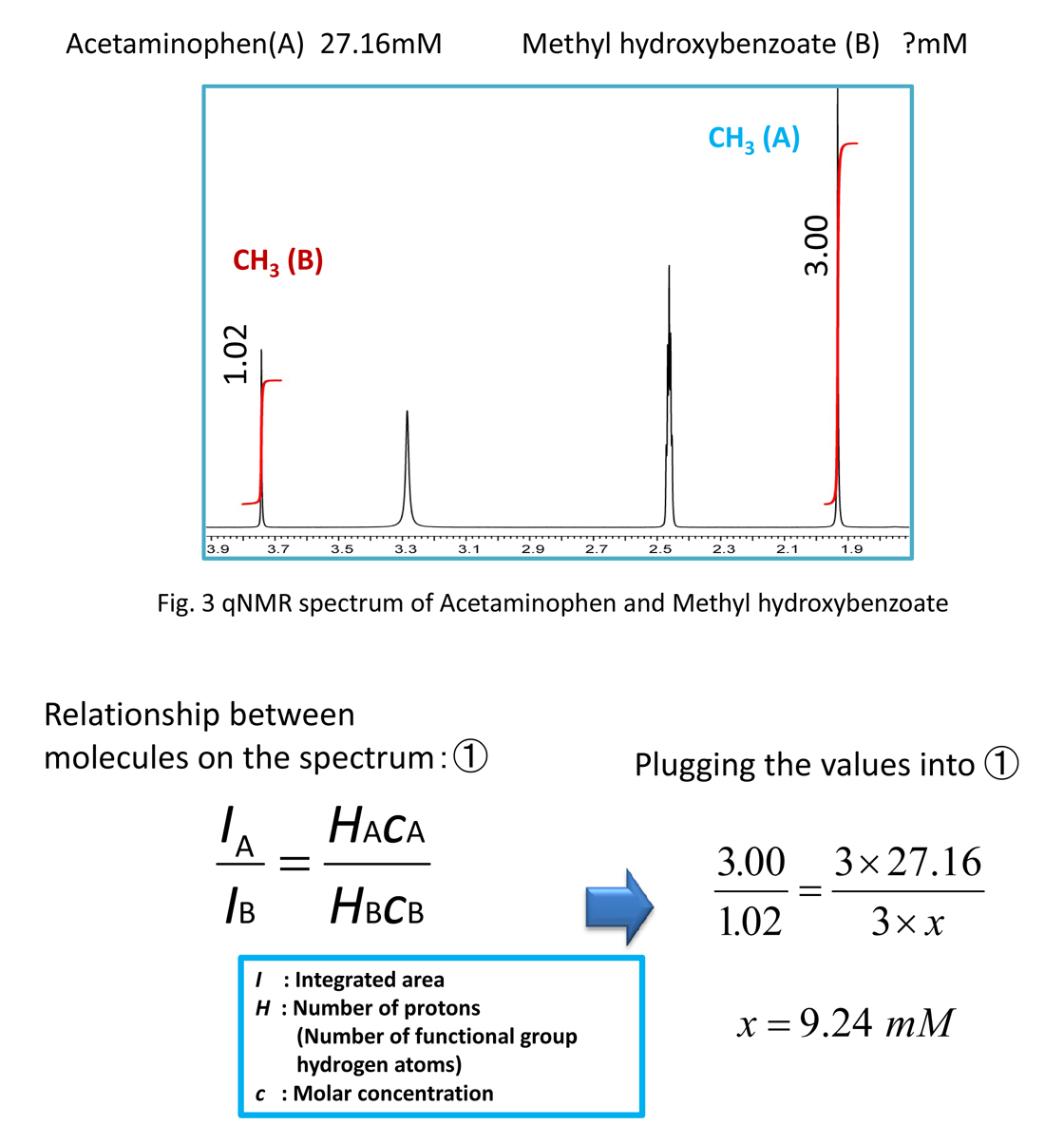
Figure 3 shows the results for a separately prepared sample.
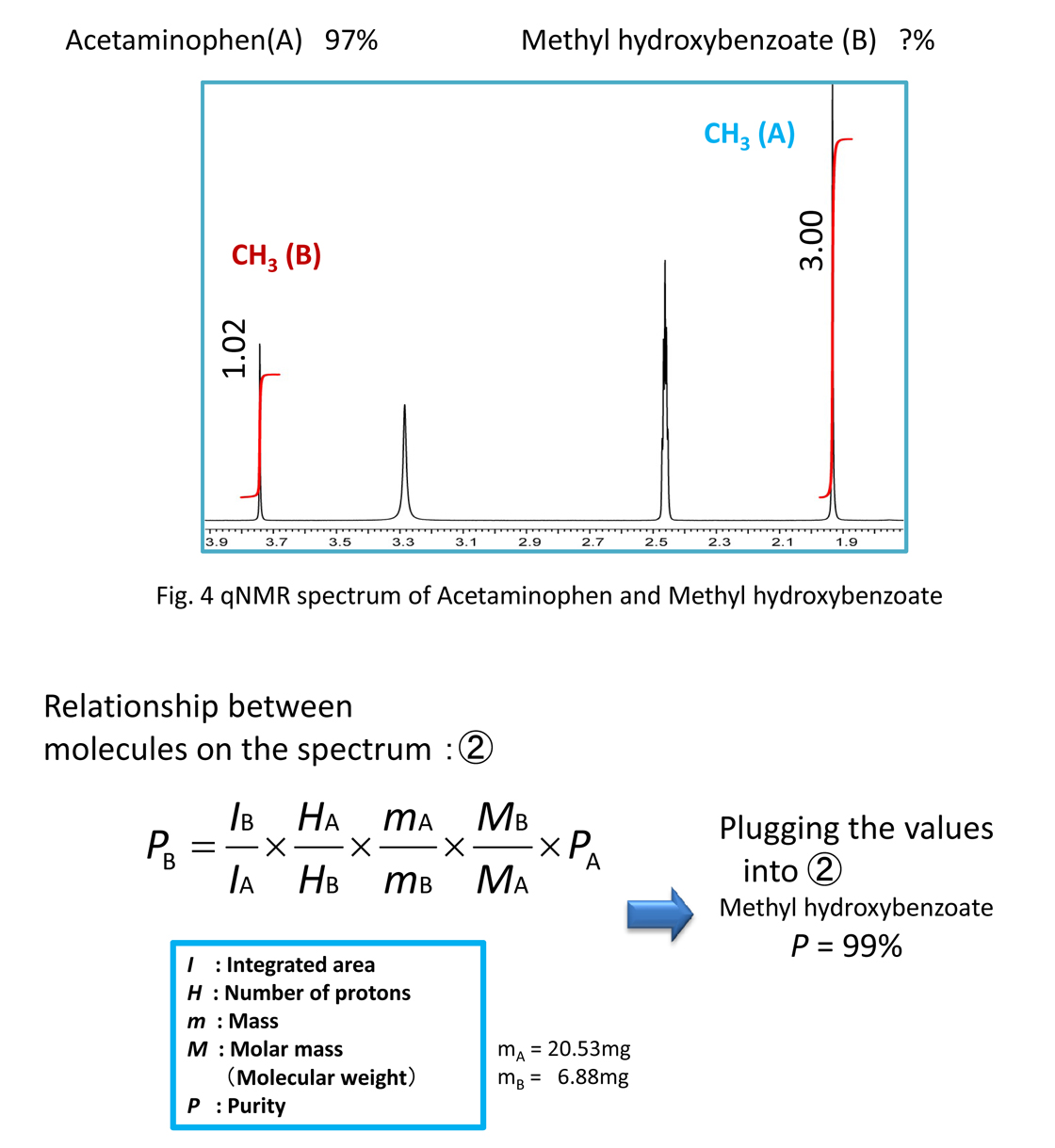
This sample was prepared by adjusting the concentrations so that the acetaminophen can be used as a quantitative standard. Based on the first equation, the relationship also holds between different molecules. Therefore, from Equation 1 we can obtain the concentration of the methyl hydroxybenzoate from the acetaminophen. Since the target molar concentration ratios become clear during the quantification, by knowing the purity of the standard substance, it is possible to determine the purity of the analyte.
Most NMR phenomena can be quantitatively expressed using quantum mechanics.
That is why, strictly speaking, the principles of quantitative analysis are somewhat
complicated, but can be represented with numerical formulas.
Reference:
U.Holzgrabe, I.Wawer, B.Diehl “NMR SPECTROSCOPY IN PHARMACEUTICAL ANALYSIS” ELSEVIER (2008)
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
