Let’s try doing some quantitative NMR (3)
Issue 10
Practical pointers for sample preparation
General measurement and Quantitative measurement
Automation of analytical instruments has advanced in recent years, and NMR is no exception. With JEOL equipment 1H, 13C and two dimensional measurements can be made just by pressing one button. However, it is important to be aware that automated measurement is not suitable for quantitative analysis by using NMR. As you know, NMR is less sensitive than other analytical instruments. For this reason the main focus of NMR technology development has been on how to raise the sensitivity. This is achieved not only by improving the performance of the instrument, but also with the measurement conditions. In any case, it must be possible to detect the signal, and that is why the default settings for the conditions in automatic measurement are optimized for signal detection, at the expense of the quantitativeness. So, how much of a sacrifice do we make for the quantitativty, in case of automation measurement? This can be illustrated with an example of an intra-molecular integral ratio. Figure 1 shows the 1H NMR spectrum of ethyl crotonate. As information for structural analysis, there is no problem to interpret this intra-molecular integral ratio as 1: 1: 2: 3: 1, but when trictly checking the data, we see that the integral values of the double bond portions are low by 7 to 8%. Therefore, just from this result alone it is clear that these are not appropriate conditions for quantitative measurement.
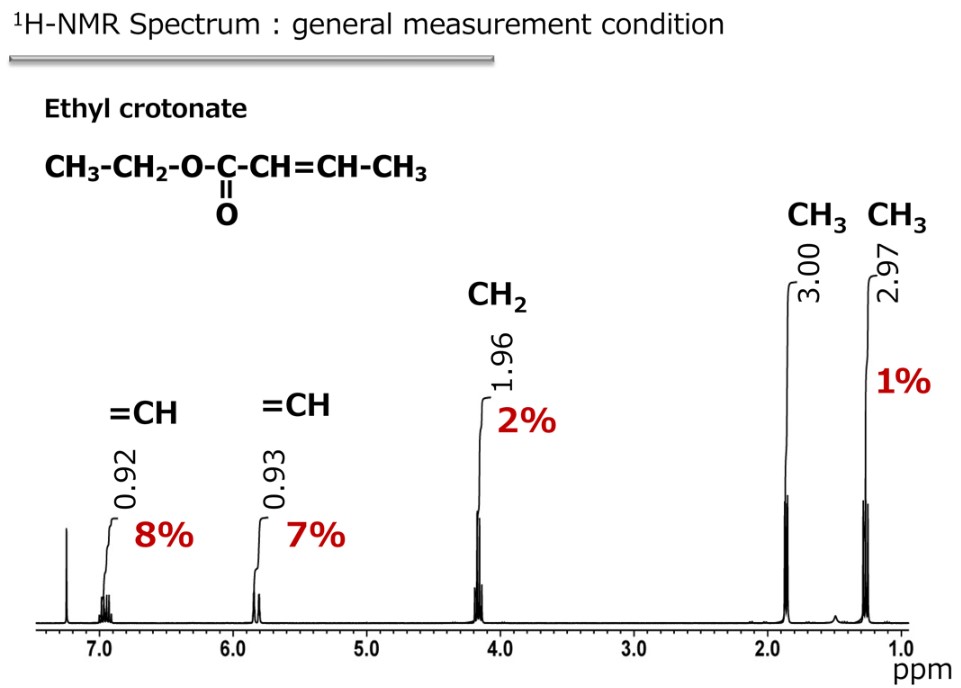
Fig. 1 Ethyl crotonate
General measurement and Quantitative measurement
There are various factors that contribute to the quantitativeness of the NMR spectrum, but just optimizing the measurement conditions can improve dramatically. Specifically, the “repetition time” in the measurement parameters is a key point. In order to increase the sensitivity of NMR measurements, data acquisition is required.
For the integration, pulses are repeatedly applied to the sample, and the “repetition time” is the length of time between the pulses. To explain this a little further, remember that in NMR, radio frequency pulses are applied to the nuclear spins within a magnetic field, and the behavior of the nuclear spins is observed. After being affected by a pulse, the nuclear spins require a certain amount of time to return to their original state. If the time allowed for returning to the original state is not sufficient, the quantitativeness of the signal diminishes as the data acquisition is repeated. This results in a spectrum with questionable quantitativeness. In other words, it is important to set a sufficiently long repetition time between pulses for quantitative measurements. Figure 2 shows the comparison with the measurement conditions that consider quantitativeness.
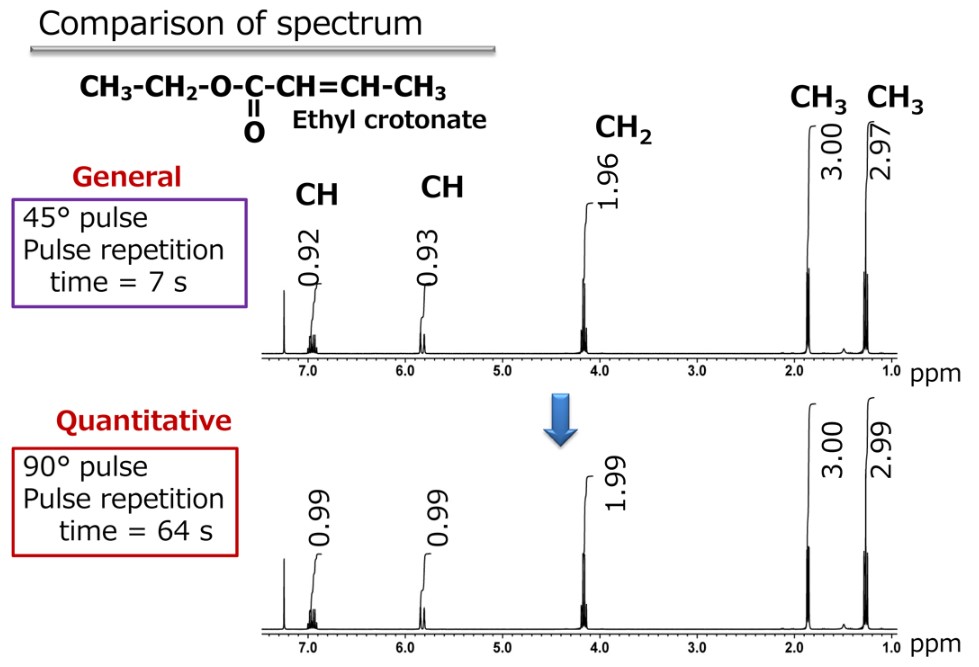
Fig. 2 Ethyl crotonate spectrum with quantitative conditions
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
