Chemical structure analysis of sulfide-based solid electrolytes by 31P solid-state NMR
NM220016
Sulfide-based solid electrolyte has been expected as an electrolyte material for the all-solid-state battery, due to its high conductivity and low interface resistance against electrodes. Lithium thiophosphate (LPS) series is a typical sulfide electrolyte, and it is known that its Li+ conductivity is greatly dependent on the crystallinity and content volume in the secondary phase. 31P solid-state NMR is a powerful measurement method that can quantitatively calculate the crystallinity and the content ratio of secondary phases. Here, an analysis example is introduced, in which structural changes of the sulfide-based solid electrolyte LPS depending on the difference of preparation methods, were analyzed by 31P solid-state NMR.
LPS structural changes depending on the difference of preparation methods - degree of crystallinity and unit structure -
Measurement sample
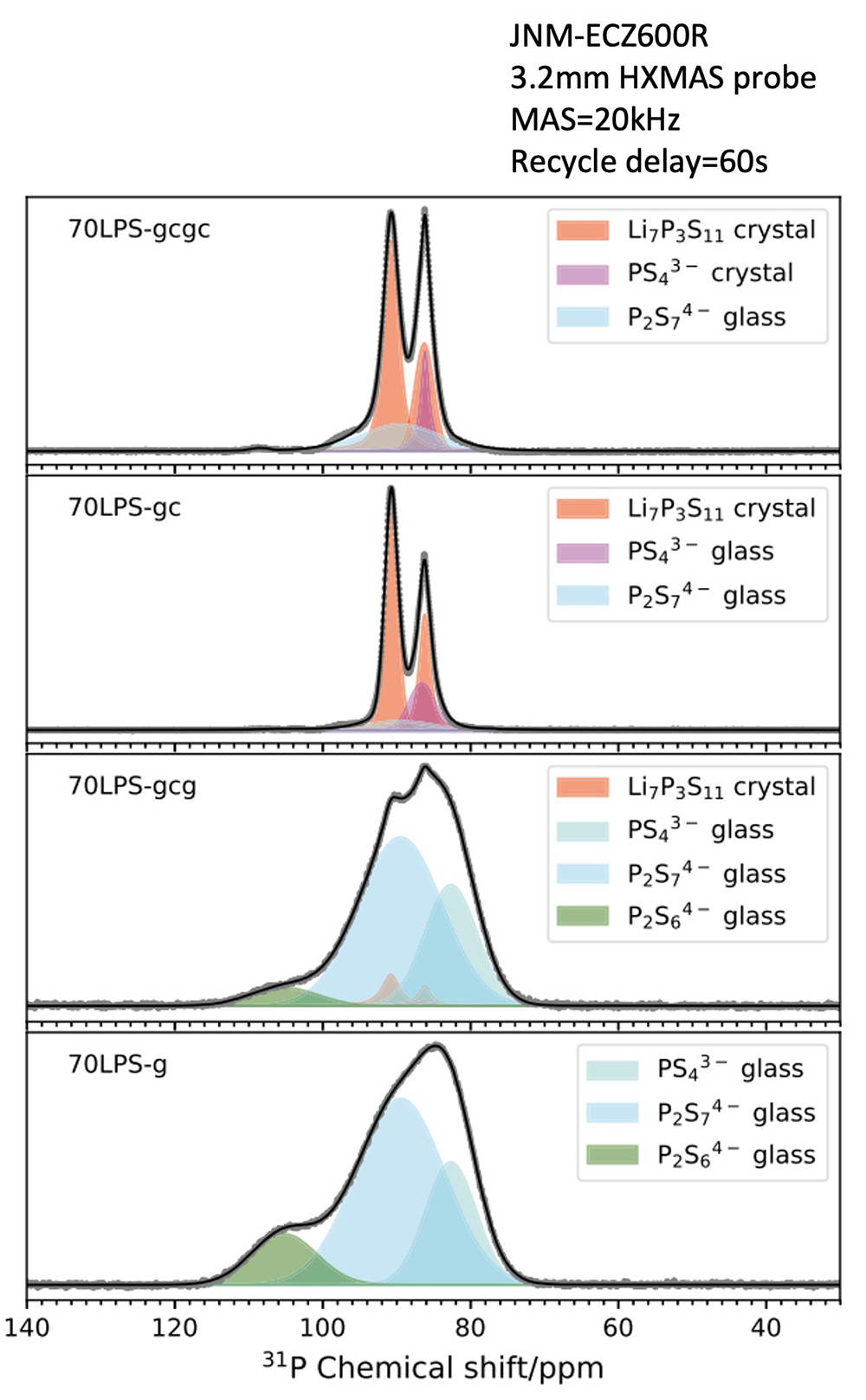
We have prepared lithium thiophosphate samples with the following four structures (70Li2S−30P2S5) and performed structural analysis.
70LPS-g: Mixture of Li2S and P2S5 was obtained with the mix ratio of 7 to 3 by the ball-mill method. Glassy state.
70LPS-gc: 70LPS-g was annealed at high temperature and crystalized.
70LPS-gcg: 70LPS-gc was vitrified (glassy state) again by the ball-mill method.
70LPS-gcgc: 70LPS-gcg was annealed again at high temperature and crystallized.
Results and Consideration
31P solid-state NMR spectra of the four samples are shown in the figure on the right. Large differences in line width in the spectra are seen between crystalline samples and glass samples. Since a crystal has uniform bond length and angle, the line width is narrow for exhibiting a linear Lorentz-type spectral shape. But for a glassy state, the bond lengths and angles are distributed, resulting in a thicker line width and a Gaussian-type spectral shape.
The spectrum of 70LPS-g can be separated into three types of Gaussian components, and it is found that the three units are constituted from the chemical shift range; PS43-, P2S74-, and P2S64-. On the other hand, in the spectrum of 70LPS-gcg, which was crystallized once and vitrified again, crystal components of Li7P3S11, which have a narrow line width, slightly appear, and the area ratio of the three units is found to change. Thus, solid-state NMR elucidates the difference of in chemical structure even in the same glassy state.
Li7P3S11 crystal consists of two units: PS43- unit and P2S74- unit. Since the number of P atoms are two coming from PS43- unit, and four coming from P2S74- unit, the area ratio of 31P signal is 1:2. With this as constraint conditions, when peak deconvolution was performed for 70LPS-gc, it was found to have glass components coming from PS43-, P2S74- units with a thicker line width and a Gaussian-type spectral shape, other than Li7P3S11 crystal.
On the other hand, 70LPS-gcgc clearly has a larger PS43- component and exhibits a Lorentzian spectral shape with a narrow line width. This result confirms that not only the Li7P3S11 crystal, but also the PS43- single-unit crystal is formed. Despite the low degree of crystallinity, the ionic conductivity of 70LPS-gcgc is 1.7 times higher than that of 70LPS-gc, suggesting that the newly-emerged PS43- unit forms a new ion conduction path.
Reference
K. Uchida, T. Ohkubo, F. Utsuno, K. Yazawa, ACS Appl. Mater. Interfaces. 2021, 13(31), 37071-37081
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
