Performance Comparison Between Two Carrier Gases on a Gas Chromatograph – Mass Spectrometer Using Blank Tube Inlet and Field Ionization
MSTips No.426
Introduction
The gas chromatograph - high resolution time of flight mass spectrometer (GC-HRTOFMS) is useful for identifying the chemical composition of synthetic compounds and for confirming the molecular weight. Field ionization (FI) is a soft ionization technique suitable for producing molecular ions. The combination of blank tube inlet, where sample is introduced from the GC into the MS by blank capillary tubing, and FI can measure samples in less time than normal GC-MS measurement. In addition, GC-HRTOFMS with FI can be automated using with autosampler.
Helium is used as a standard carrier gas for GC separations, however, in recent years, helium supply has become more scarce, resulting in higher prices. As an alternative, N2 or H2 gas can be used instead of He. Nitrogen is plentiful, inexpensive, and safer than H2.
In this study, a blank tube inlet/FI technique using He and N2 as carrier gases was used to identify the chemical composition of an organic synthetic compound and confirm the molecular weight distribution.
Measurement
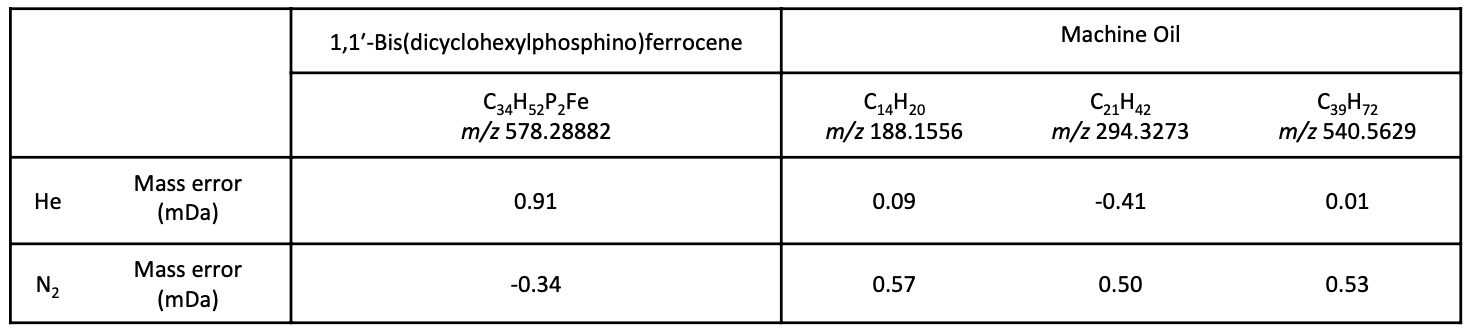
Samples tested were 1,1′-bis(dicyclohexylphosphino)ferrocene for identifying the chemical composition, and commercial machine oil for observing molecular weight distribution. 1,1′-bis(dicyclohexylphosphino)ferrocene was prepared as 1 mg/mL and machine oil was prepared as 10mg/mL, both in n-hexane.
Table 1 shows the GC/HRTOFMS measurement conditions. In the case of using blank tube, it was not necessary to set the carrier gas flow rate for appropriate linear velocity, and was set to a value that preserved the vacuum of the MS while allowing pressure regulation at the GC inlet. The calibrant for drift compensation was introduced from the standard sample reservoir after samples were detected.
Table 1. Measurement conditions
Results
Total ion current chromatograms (TICCs) and FI mass spectra of 1,1′-bis(dicyclohexylphosphino)ferrocene are shown in Figure 1. The molecular ion [M]+・ was detected under both carrier gas conditions. No notable differences in isotope patterns of the molecular ion were observed between the two carrier gases. Measurement time using each carrier gas was less than 1 minute. Table 2 shows mass errors between measured m/z and calculated m/z. No differences in mass accuracy caused by carrier gas were observed.
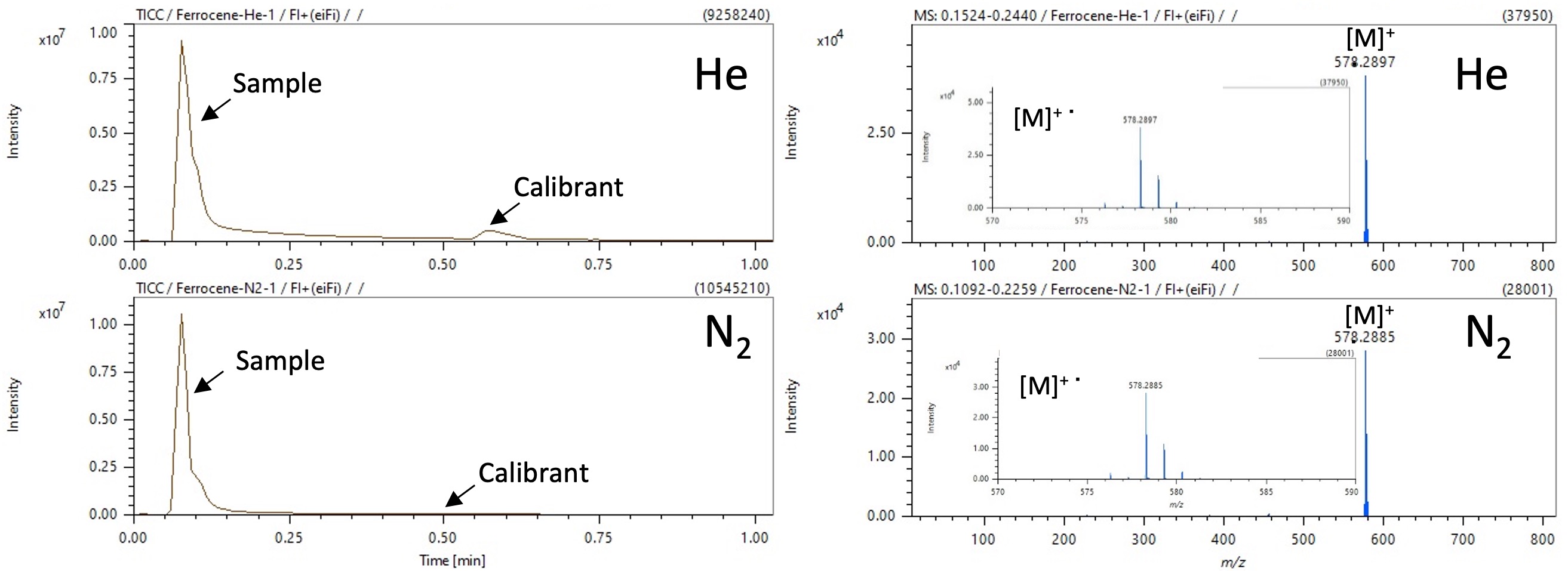
Figure 1. TICCs and FI mass spectra of 1,1ʹ-bis(dicyclohexylphosphino)ferrocene under helium and nitrogen carrier gas conditions
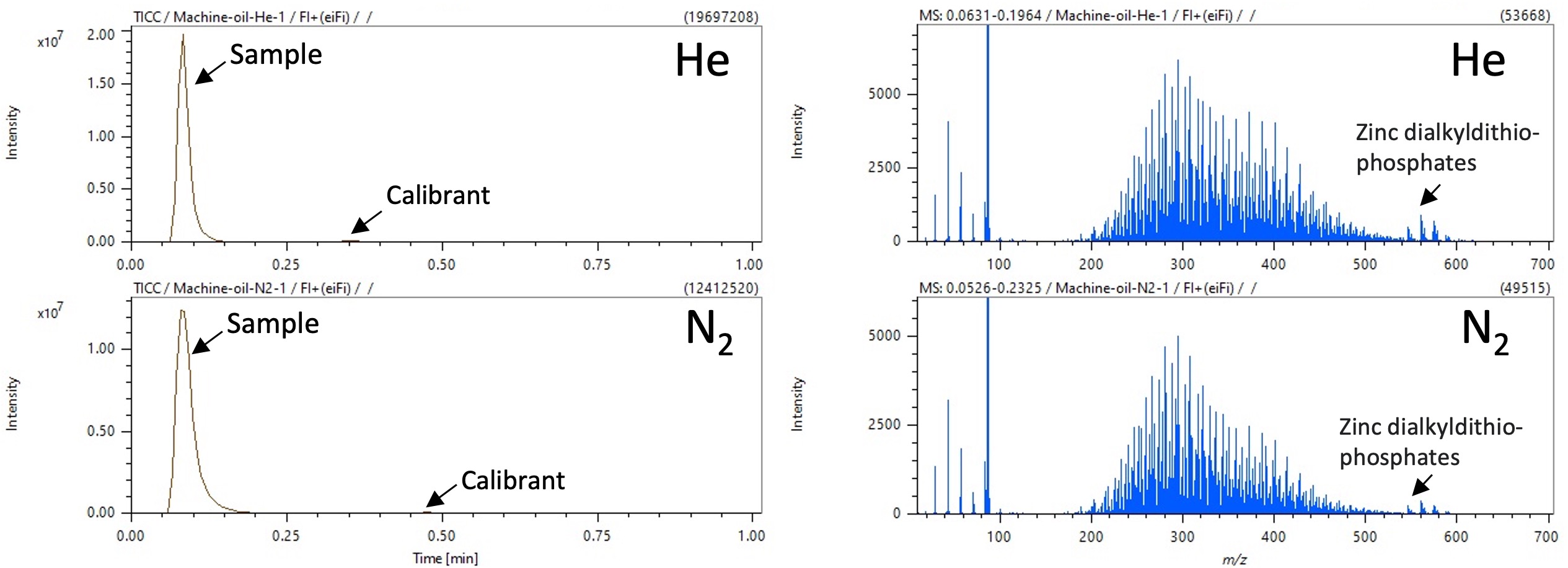
Figure 2. TICCs and FI mass spectra of machine oil using helium and nitrogen carrier gas condition
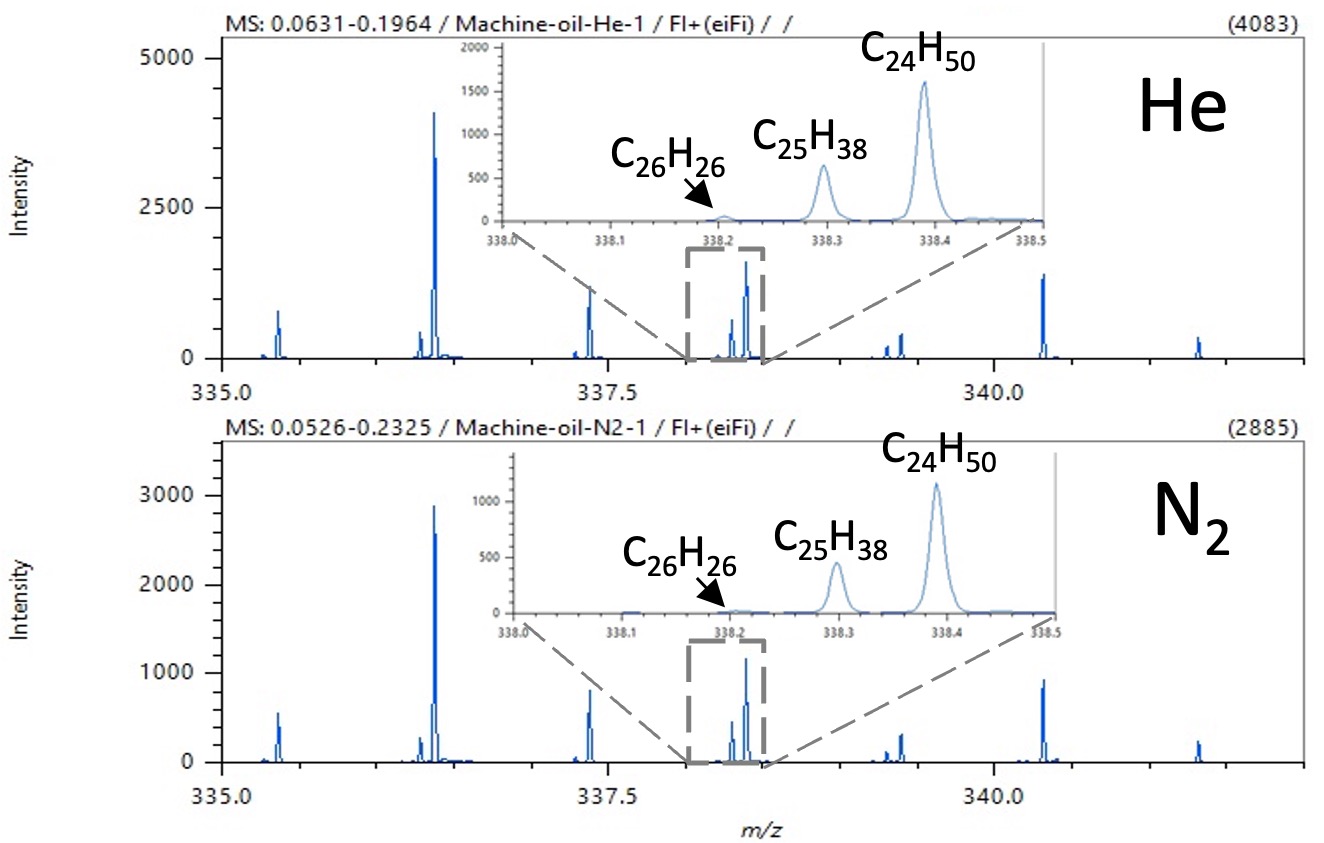
Figure 3. Enlarged FI mass spectra shown in Figure 2
(Around m/z 335 ~ 342)
Results for the machine oil sample are shown in Figure 2. The molecular ion [M]+・ was detected under both carrier gas conditions. Mass spectra for the machine oil consisted of hydrocarbons, which showed a molecular distribution around m/z 188~540, and zinc dialkyldithio-phosphates, which were observed at m/z 546~616. The mass errors at m/z 188, 294 and 540 (corresponding to the low molecular weight side, the base peak, and the high molecular weight side of the mass distribution, respectively) are shown in Table 2. No notable differences in mass accuracy caused by carrier gas were observed, and comparable results were obtained from both carrier gases.
Figure 3 shows an enlarged view of the FI mass spectra shown in Figure 2 around m/z 335-342 and around m/z 338.
Three m/z signals were confirmed within 0.5 u around m/z 338. Not only were these three signals completely mass-separated, but their chemical composition could be estimated.
Table 2. Comparison of molecular ion mass errors between He and N2 carrier gas conditions
Summary
In this application note, we compared two carrier gases measurement results using a GC-HRTOFMS and blank tube inlet/FI technique. The results show comparable mass accuracy, molecular weight distributions, and mass resolution by both carrier gases. Therefore, N2 gas has an advantage for cost.
The GC-HRTOFMS with blank tube inlet/ FI technique can be used as powerful tool for identifying the chemical composition and molecular weight regardless of whether He or N2 is used as a carrier gas.
Reference
1) Masaaki Ubukata, Akihiko Kusai, Junichi Osuga, Kazuo Tanaka, Takeo Kaneko, Kensei Kobayashi., J.Mass Spectrom. Soc. Jpn., 56(1), 13-19(2008).
Solutions by field
Related products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.

