Fast MAS solid-state NMR toolbox for biomolecules
NM210002E
Solid-state NMR is a useful tool to elucidate protein structures. Although fully 13C and 15N labelled samples are required, solid-state NMR provides complementary or unique information that is not accessible by other typical methods. For example, there are, in principle, no upper or lower limits to molecular weight in solid-state NMR, unlike solution NMR and cryo EM. In addition, solid-state NMR is a very sensitive probe of local dynamics. One of the stumbling block of solid-state NMR is sample amount. Indeed, 20 – 50 mg of fully labelled (expensive!) samples are required for 3.2 mm / 4 mm MAS rotors. However, the recent developments allowing MAS rates above 70 kHz change the situation completely. [1] Indeed, the 0.75 mm (1 mm) MAS rotors used for fast MAS experiments (up to 120 (80) kHz) only require 290 (800) nL of sample, enabling solid-state NMR measurements on sub-milligram amounts of sample. Another great advantage of fast MAS is the averaging out of most of the anisotropic interactions, allowing the use of low power schemes (decoupling, recoupling, cross-polarization etc.), which in turns avoids sample heating. High resolution 1H spectra become readily available in these conditions, especially for microcrystalline proteins. This is the biggest advantage of fast MAS, since 1H detection dramatically improves sensitivity compared to traditional 13C detection. As a consequence, fast MAS solid-state NMR is now a versatile tool to study the structure and dynamics of biomolecules, for which the first step is sequence-specific resonance assignment. To this end, requisite experiments for sequential backbone and sidechain assignments are well established. [2] Here, we introduce the tool box available for the Delta software together with examples on fully protonated Het-s (226-280) protein.
[1] Review: Y. Nishiyama, Solid State Nucl. Magn. Reson. 78 (2016) 24-36.
[2] E. Barbet-Massin et al., J. Am. Chem. Soc. 136 (2014) 12489-12497.
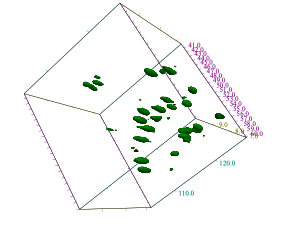
Fig. 1 3D hCaNH spectrum of Het-s (226-280) measured using 1mm HCN MAS probe with JNM-ECZ900R spectrometer at 21.1 T. MAS rate is set to 70 kHz.
Data courtesy of Prof. Antoine Loquet (Université Bordeaux) and Dr. Yusuke Nishiyama (RIKEN)
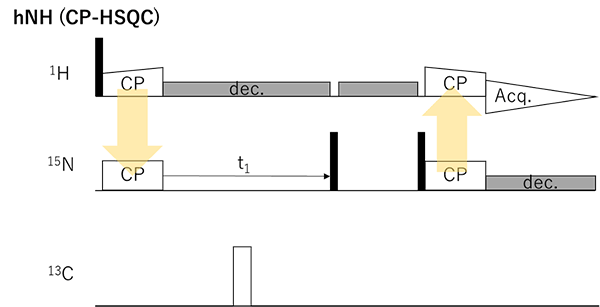
Fig. 2 Basic 2D 1H/15N correlation experiments. Filled and open rectangles represent π/2 and π pulses, respectively.
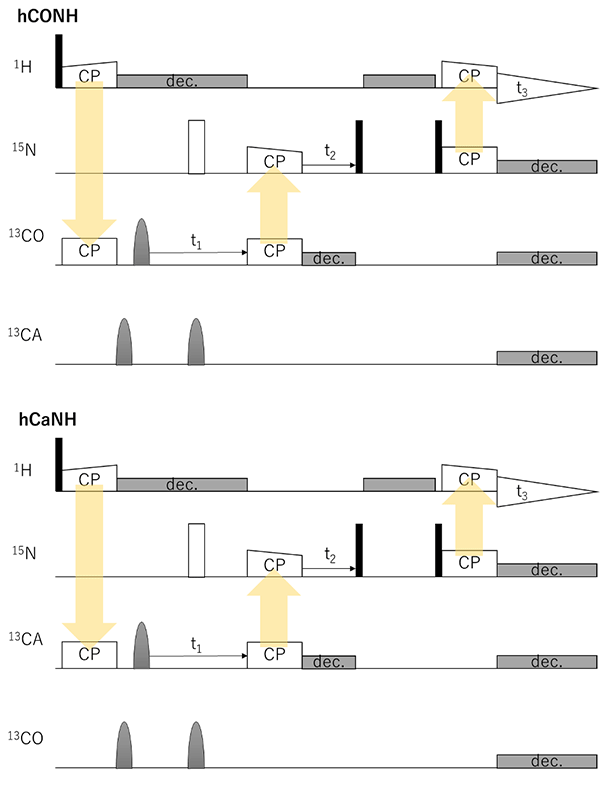
Fig. 2 (cont.) hCONH and hCaNH to probe bonded 13C/15N/1H connectivities.
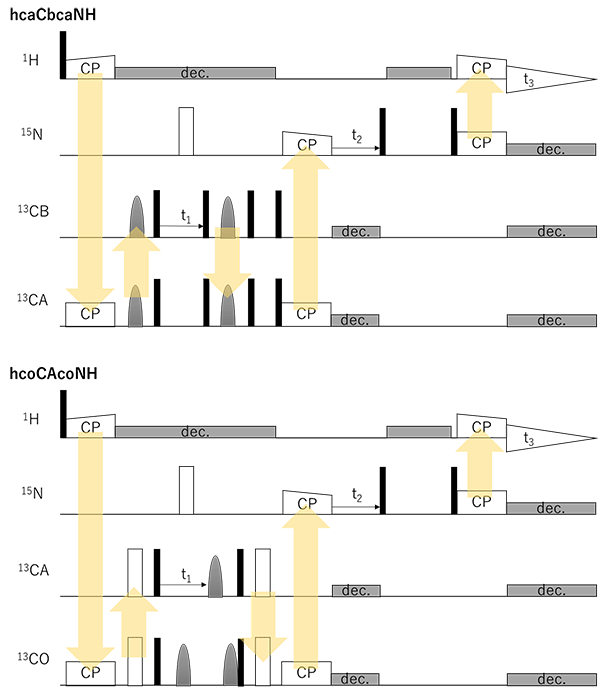
Fig. 2 (cont.) hcaCbcaNH and hcoCAcoNH experiments for sidechain and backbone assignments. [3]
[3] E. Barbet-Massin et al., J. Biomol. NMR 56 (2013) 379-386.
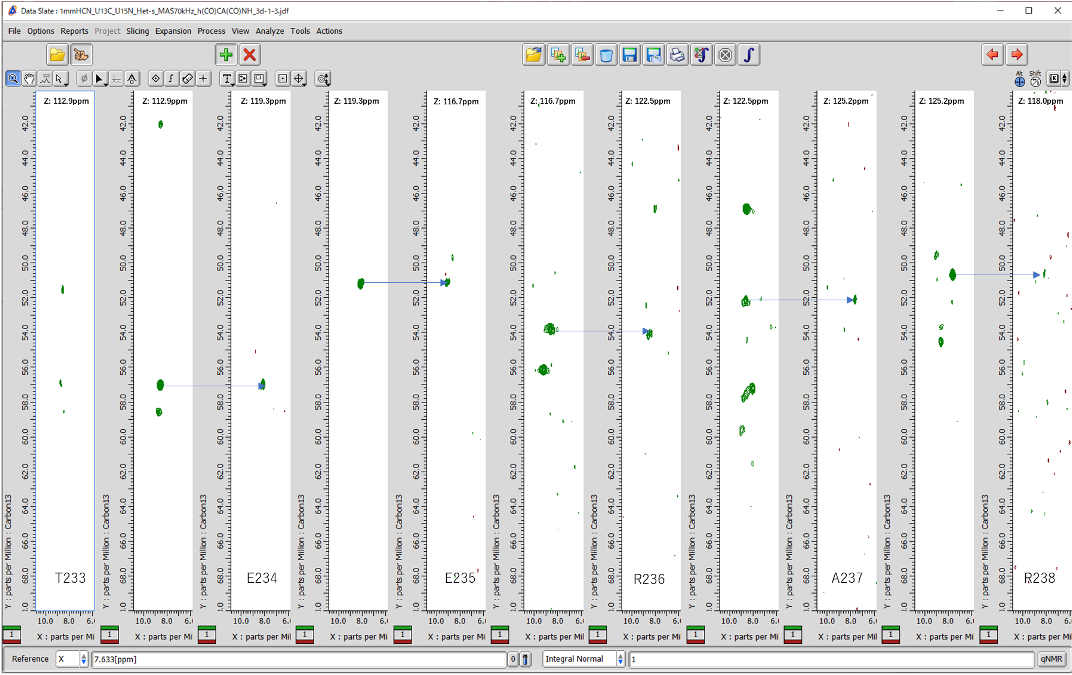
Fig. 3 Sequential assignment of backbone signals of Het-s (226-280) measured using 1mm HCN MAS probe with JNM-ECZ900R spectrometer at 21.1 T. MAS rate is set to 70 kHz. CH stripes of hCaNH and hcoCAcoNH spectra are shown.
Data courtesy of Prof. Antoine Loquet (Université Bordeaux) and Dr. Yusuke Nishiyama (RIKEN)
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 1,740.8KB
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
