Probing hydrogen bond network in pharmaceutical samples by using ultra fast MAS 1H solid state NMR
NM180004
For pharmaceutical formulation developments, probing binding form of a cocrystal between an active pharmaceutical ingredient (API) and a coformer is of great importance not only for realizing its properties but also for the intellectual property protection point -of-view. 1H solid state NMR using ultra fast MAS can be a powerful tool to probe hydrogen bond (HB) network and differentiate salts and cocrystals.
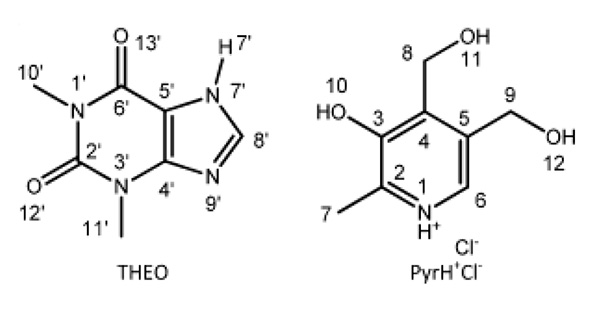
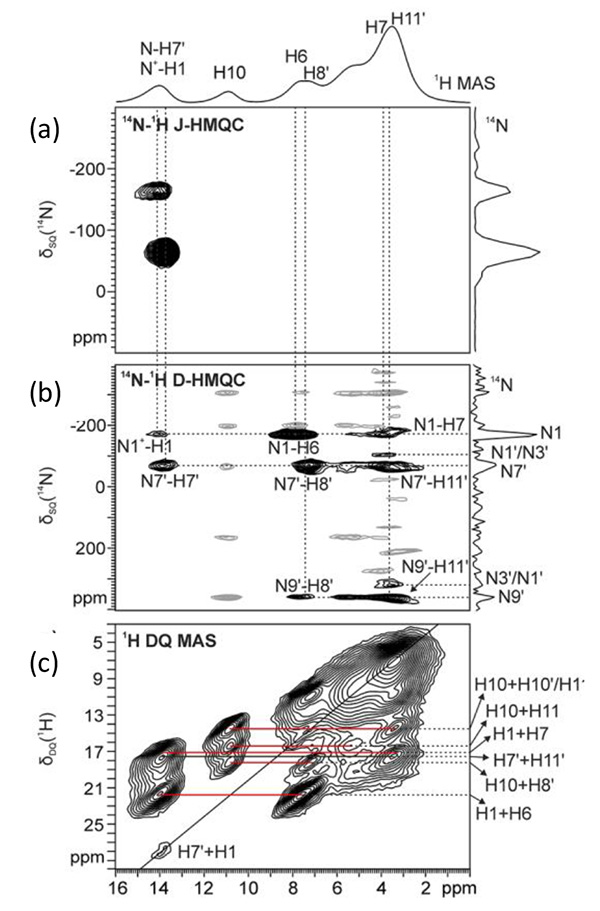
We report here an example of HB network analysis for a codrug formed by two APIs: namely theophylline (THEO) and pyridoxine·HCl (PyrH+Cl-). 1 The HB characterization of the codrug is challenging, since five HB donors (three OH and two NH groups) and three acceptors (two C=O groups and one aromatic nitrogen) are present (Fig.1). In fact, single crystal X-ray diffraction could not access it due to the poor definition of the hydrogen atom positions. In order to probe the HB network and define binding form properly, we used two ver sions, J and D, of 14N-1H HMQC. 2-4 The J-version is a simple HMQC experiment with rotor syncronous 14N observation in the indirect dimension through J-coupling and residual dipolar splittings (RDS), and mainly gives information of 14N-1H covalent bond. On the other hand, the D-version utilizes 14N-1H dipolar coupling reintroduced by a symmetry-based sequence (SR4), and observes longer-range N···H proximities. The combination of the two methods allows defining of the ionic character of the codrug, namely, salt or cocrystal.
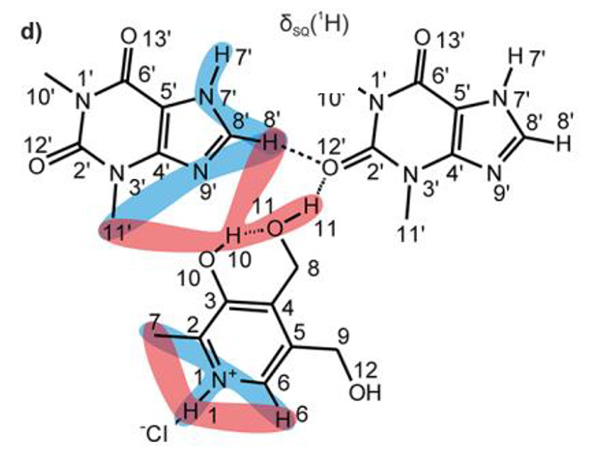
The 14N-1H J- and D-HMQC spectra are shown in Fig.2a and 2b, respectively. The J-version spectrum shows only two correlation peaks, corresponding to covalently bonded N-H atoms (i.e., N1+-H1 and N7’-H7’). This suggests that there is no proton transfer between THEO and PyrH+Cl-, thus the codrug can be more properly defined as a salt co-crystal due to the ionic character of PyrH+Cl-. On the other hand, the D-version shows several correlations regarding with longer range 14N···1H proximities. Combination with 1H DQ MAS spectrum, which gives 1H-1H proximity information, elucidates the HB network described in Fig.3. This structure is supported and competed by X-ray data and DFT calculations.
(Reprinted with permission from ref 1. Copyright at 2018 American Chemical Society)
(Reprinted with permission from ref 1. Copyright at 2018 American Chemical Society)
References
- F. Rossi, P. C. Vioglio, S. Bordignon, V. Giorgio, C. Nervi, E. Priola, R. Gobetto, K. Yazawa, and M.R.Chierotti, Cryst. Growth Des., 2018, 18 (4), 2225–2233
- S. Cavadini, A. Lupulescu, S. Antonijevic, and G. Bodenhausen, J. Am. Chem. Soc. 2006, 128, 7706
- Z. Gan, J. Am. Chem. Soc. 2006, 128, 6040
- Y.Nishiyama, Y. Endo, T. Nemoto, H. Utsumi, K. Yamauchi, K. Hioka, and T. Asakura, J. Magn. Reson. 2011, 208, 44-48
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 594.7KB
SEARCH APPLICATIONS
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
