Microstructures of Vinyl Ester Resins Clarified by Solid-state 13C Chemical Shifts and 13C T1ρ Relaxation Times
NM140006E
Vinyl ester resins are used as adhesives and insulators of electrical equipment, and their novel polymerization methods are investigated for their higher thermostability. It was reported [1] that the living radical polymerization of vinyl ester monomers (VE) and styrene monomers (ST) with the initiator of alky borane DEMB (diethylmethoxyborane) improves the thermostability of vinyl ester resins compared with those obtained with the conventional initiators of AIBN (azobisisobutylonitril) and DBPC (1,1-di(t -butylperoxy)cyclohexane). In addition to the reported macroscopic physical properties such as thermal weight loss, the information of the microscopic structures of the materials may play an important role for understanding the physical properties and further improvement of the materials. This note introduces a research on the different microstructures of vinyl ester resins produced with various polymerization initiators studied by 13C NMR [2].
Figure shown below are the 13C CPMAS spectra of vinyl ester resin produced with the initiator DBPC (DBPC-VEST) and that with DEMB (DEMB-VEST). Almost all the lines are assigned to the carbons of the plausible structures of these vinyl esters.
13C CPMAS spectra
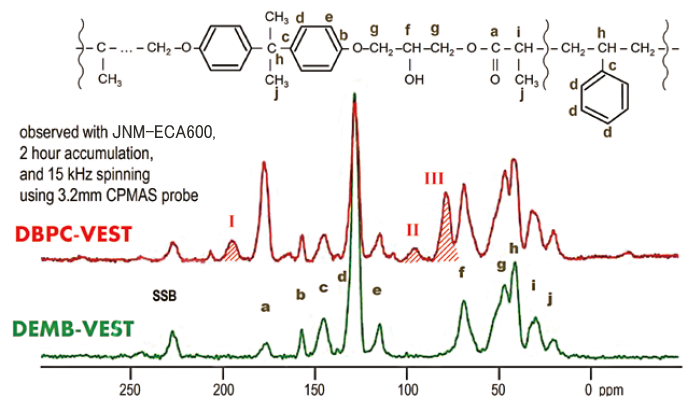
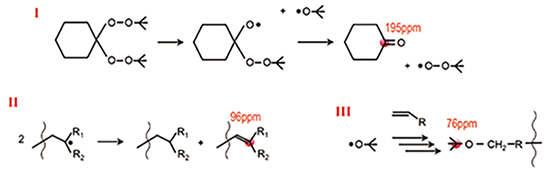
On the other hand, three additional lines (I, II, III) are detected for DBPC-VEST compared with DEMB-VEST. These lines are considered to be assigned to decomposition of DBPC (I), disproportionation of vinyl compound derivative (II), and polymerization addition reaction (III). Especially on disproportionation (II), the spectrum of DEMB-VEST shows that its terminal double bonds are much less than those of DBPC-VEST, consistent with the result of thermal weight loss.
We measured the 13C T1ρ (longi-tudinal relaxation time in the rotating frame) and list the results in Table. The values of 13C T1ρare found to greatly decrease in the order of DBPC > AIBN > DEMB.
In general, 13C T1ρ values de-crease when exist many of micro- scopic molecular motions whose speed matches with the applied spin-lock RF strength (30 kHz in this study). Table indicates that such slow motions occur in DEMB- VEST very frequently.
Also, T1ρas well as T2 is known to relate with the density of microscopic cross linkings; the larger cross-linking density materials have, the shorter their T1ρ(and T2 ) becomes [3]. The above Table suggests that the cross-linking densities become larger in the order of DBPC < AIBN < DEMB, and this fact is consistent with the physical properties of glass-transition temperatures and elastic modulus of rubber phase [1].
| Carbon# | Chemical Shift (ppm) |
T1ρ (13C) (ms) | ||
| DBPC-VEST | AIBN-VEST | DEMB-VEST | ||
| a | 178.0 | 11.5 | 30.4 | 10.2 |
| b | 157.1 | 58.1 | 29.1 | 11.5 |
| c | 144.4 | 31.8 | 21.9 | 8.2 |
| d | 128.1 | 8.5 | 6.4 | 1.6 |
| e | 114.7 | - | 5.1 | 2.4 |
| f | 68.7 | 4.7 | 4.6 | 0.7 |
| h | 46.1 | 10.1 | 9.6 | 2.9 |
| i | 31.0 | 16.1 | 12.9 | 5.1 |
| j | 19.7 | 16.5 | 13.6 | 6.0 |
Thus, NMR is prospective to clarify the origin of macroscopic physical properties and may provide the microscopic information useful for materials developments.
※The experimental results were obtained by cooperation with Hitachi Ltd. and Hitachi Power Solutions Co., Ltd.
References
[1] T. Muraki, S. Amou, H. Morooka, H. Kagawa, K. Souma, Network Polymer, 34(4), 178 (2013).
[2] Y. Kajihara, T. Muraki, Network Polymer, 35(4), 161 (2014).
[3] K. Fukumori, Toyota Central Labs. R&D Review, 28, 11 (1993).
SEARCH APPLICATIONS
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
