Protein-Ligand Interaction I: STD experiments
NM130007E
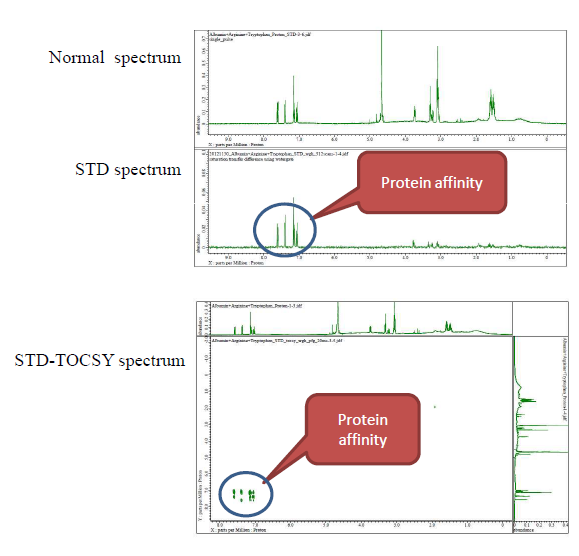
Saturation Transfer Difference (STD) NMR spectroscopy is a very useful tool for screening compounds for protein-binding activity. 1.2) In this method, the magnetization from proteins transfers to all compounds in solution, but only the compound with binding activity will be saturated due to the protein-ligand interaction. The difference between a saturation transfer difference spectrum and a normal NMR spectrum can clearly identify binding-activity compounds. Recently we have used this method on albumin, L-(+)-arginine and L-tryptophan as standard experiments on a JEOL Resonance JNM-ECS 400MHz spectrometer.
Here we show the results of 1D and 2D STD experiments.
Referene
- (1)Moriz Mayer and Bernd Meyer, Angew. Chem. Int. Ed. 1999, 38, 1784-1787.
- (2)Moriz Mayer and Bernd Meyer, J. Am. Chem. Soc. 2001, 123, 6108-6117.
SEARCH APPLICATIONS
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
