Magnetic nanoparticles and superparamagnetic resonance (1) "Size effects"
ER210005E
Bulk crystals (> 100 nm) of metal and semiconductors have electrical conductivity that is derived from free electrons, which belong to the continuous energy levels called "band structure". When these crystals are downsized to the mesoscopic region (Fig.1 : 1 – 100 nm), the space of energy levels is no longer regarded as "continuous", but discrete. Consequently, it shows new physical properties that are different from the bulk, atoms and molecules. This is called quantum size effect[1].
Triiron tetraoxide (Fe3O4), which is ferrimagnet as well as half metal (or semiconductor), is well known as "magnetite", and is a historically familiar compound. Fine particles of magnetite, as well as other metals, show characteristic physical properties based on the quantum effect. Magnetic particles of mesoscopic size have a single magnetic domain, and behave as "superparamagnets" no longer the ferrimagnets by the effects of Brownian motion and Neel relaxation. Different from ferromagnets/ferrimagnets, superparamagnets do not have hysteresis and its magnetic anisotropy is small. Nevertheless, it has large magnetic moments similar to a ferrimagnet. Magnetic nanoparticles like magnetite are applied to the fields of Hyperthermia[2][3], Magnetic Particle Imaging[4] and as contrast agents[5] of Magnetic Resonance Imaging.
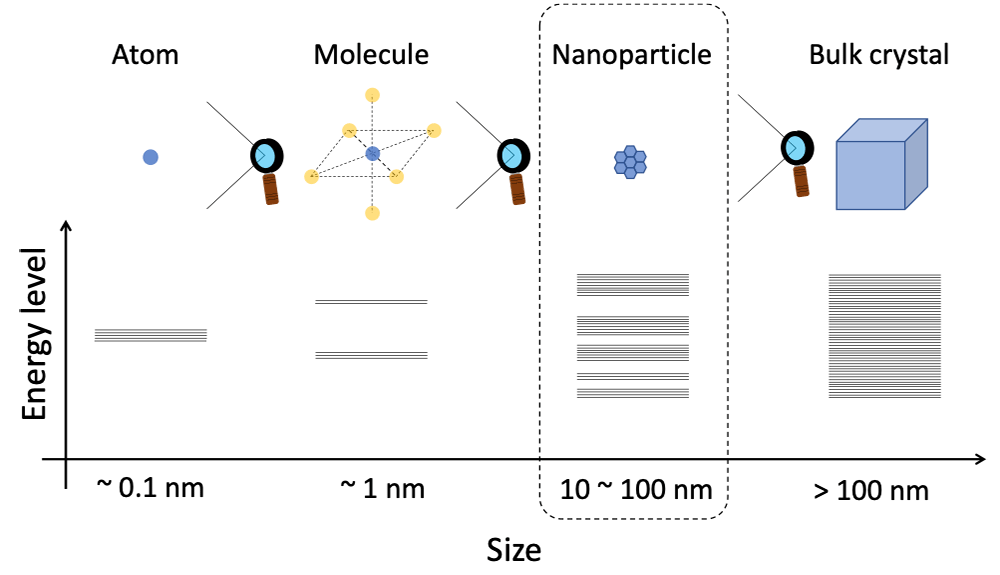
Fig. 1 A diagram that expresses the size and energy structure of conductive matter.
Variation of FMR/SPR spectra that depend on particle size
Figure 2 shows ferromagnetic resonance (FMR) spectrum of Fe3O4 powder with a diameter of 50 – 100 nm and superparamagnetic resonance (SPR) spectra of Fe3O4 magnetic nanoparticles dispersion in toluene. Large size magnetic powder has electrical conductivity as well as magnetic anisotropy. Therefore, its spectrum is observed as a broad Dysonian shape. On the contrary, magnetic anisotropy of nanoparticles becomes smaller and smaller according to downsizing, and additionally a new narrow isotropic signal appears. These spectral patterns are completely different from the large size powder and paramagnetic iron ions. This is a unique electron state dependent on the size.
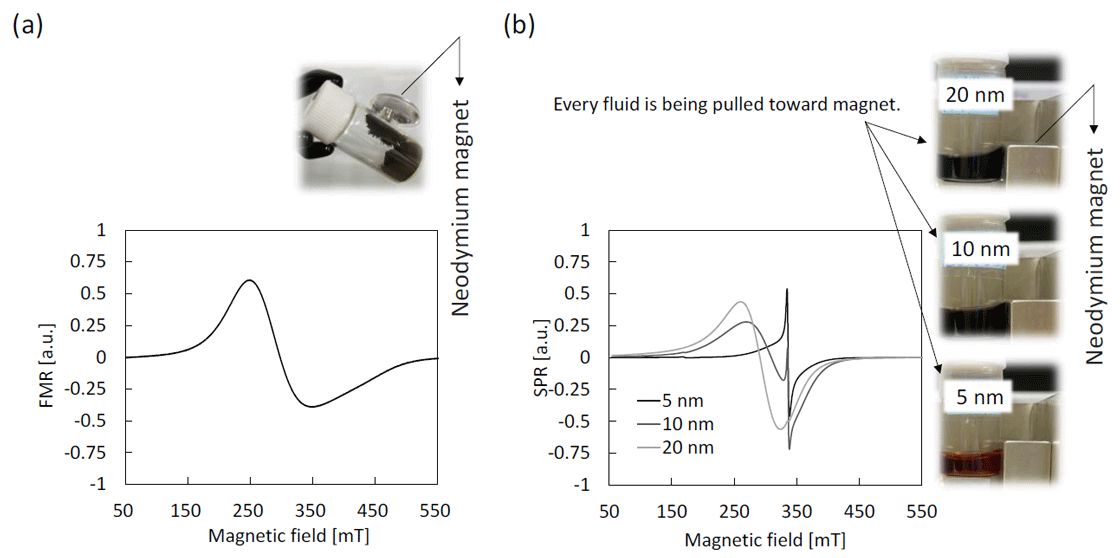
Fig. 2 ESR spectra of magnetite (Fe3O4) particles with different diameters.
(a) FMR spectrum of Fe3O4 powder with diameter of 50 – 100 nm.
(b) SPR spectra of Fe3O4 magnetic nanoparticles dispersion in toluene (0.625 mg / mL).
Reference:
- [1] R. Kubo, J. Phys. Soc. Jpn., 17, 975 (1962).
- [2] A. Rajan, N.K. Sahu, J Nanopart Res. 22, 319 (2020).
- [3] T. Morino, T. Etani, T. Naiki, N. Kawai, T. Kikumori, Y. Nishida, N. Yamamoto, T. Yasui, Thermal Med, 35 (3), 23-32 (2019).
- [4] B. Gleich, J. Weizenecker, Nature 435, 1214–1217 (2005).
- [5] R. Qiao, C. Yang and M. Gao, J. Mater. Chem., 19, 6274–6293 (2009).
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 315.9KB
SEARCH APPLICATIONS
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
