Identification and distribution analysis of additives in a molded nitrile rubber (NBR) by PY/GCxGC/HRTOFMS
MSTips No.289
Introduction
The characteristics of polymeric materials vary depending on the additives. Consequently, a variety of additives can be added into the raw polymer resin in order to achieve the required characteristics for the product. GC/MS combined with pyrolyzer system (PY/GC/MS) is used as typical analytical method for polymeric material. However PY/GC/MS is often insufficient chromatographic separation of additives and thermal decomposition compounds. And sometimes it is difficult to assign compound identities because of poor chromatographic separation. The comprehensive two-dimensional gas chromatography/high resolution time-of-flight mass spectrometry (GCxGC/HRTOFMS) is known as the technique that can be take high chromatographic separation by two different polarity GC columns and can be take accurate mass measurements used to estimate elemental compositions. It means that the GCxGC/HRTOFMS combined with PY system has a possibility to take the more detail and accurate information about polymeric materials than conventional PY/GC/MS system.
In this application note, we identified the additives and their distribution within a commercially available molded polymer by using GCxGC/HRTOFMS with pyrolysis (PY/GCxGC/HRTOFMS).
Experimental
Commercially available X-ring rubber band made of NBR was applied the measurement as test sample. A 0.5mg potion of the X-ring sliced at 1mm thickness was directly measured using PY/GCxGC/HRTOFMS system without any sample preparation. JMS-T200GC "AccuTOF™ GCv 4G" as HRTOFMS, KT2006(ZOEX Corporation) as GCxGC and PY-2020iD(Frontier Laboratories Ltd.) as pyrolyzer are used for the analysis. The details of measurement conditions for each equipment were summarized in Table 1.
Table 1 Measurement Conditions
| Instrument | JMS-T100GCV "AccuTOF™ GCv 4G" (JEOL ltd.) KT2006 (GCxGC module , ZOEX corporation) PY-2020iD (Frontier Laboratories Ltd.) |
|---|---|
| Pyrolysis conditions | |
| PY temp. | 600°C |
| PY-GC-ITF temp. | 350°C |
| GCxGC conditions | |
| Inlet temp. | 350°C |
| Inlet mode | Split mode (200 : 1) |
| 1st Column | BPX-5 (30m x 0.25 mm, film thickness 0.25 μm, Trajan SGE) |
| 2nd Column | BPX-50 (2m x 0.1 mm, film thickness 0.1μm, Trajan SGE) |
| Oven temp. program | 50°C (3min) → 5°C/min → 360°C (3min) |
| Carrier gas flow | 1.33mL/min (He, Constant flow) |
| Modulation period | 10 sec |
| MS conditions | |
| Ionization mode | EI(+): 70eV, 300μA, FI(+): -10kV, Carbon emitter |
| Interface temp. | 300°C |
| Ion source temp. | EI: 280°C, FI: OFF |
| Spectrum recording interval | 50Hz (0.02sec/spectrum) |
| m/z range | 30 – 600 |
| Drift compensation | m/z 207.0329 (C7H21O4Si4) |
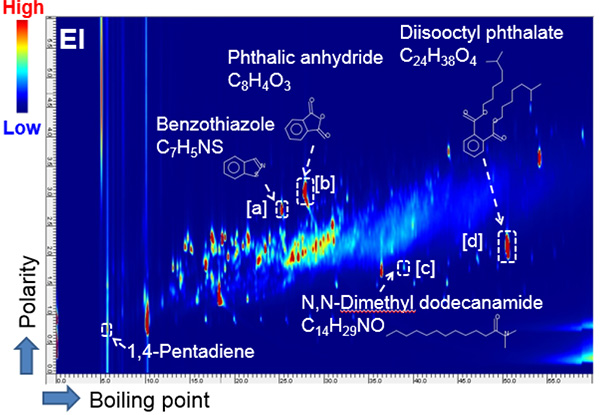
Fig. 1 2D map (EI, TICC) for x-ring
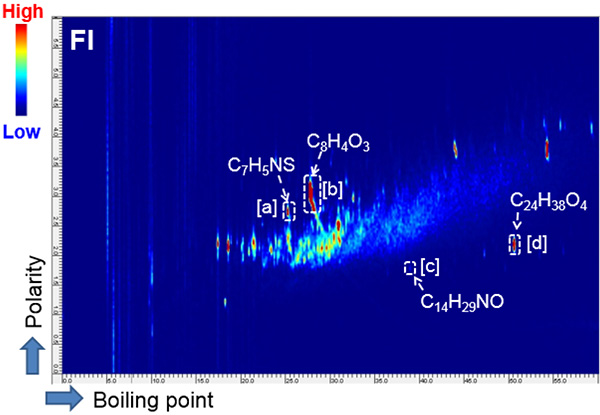
Fig. 2 2D map (FI, TICC) for x-ring
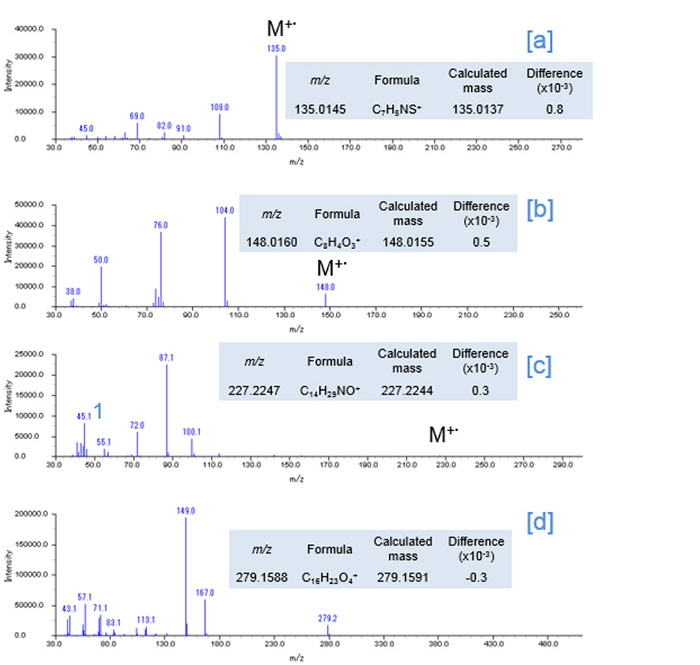
Fig. 3 EI Mass spectra
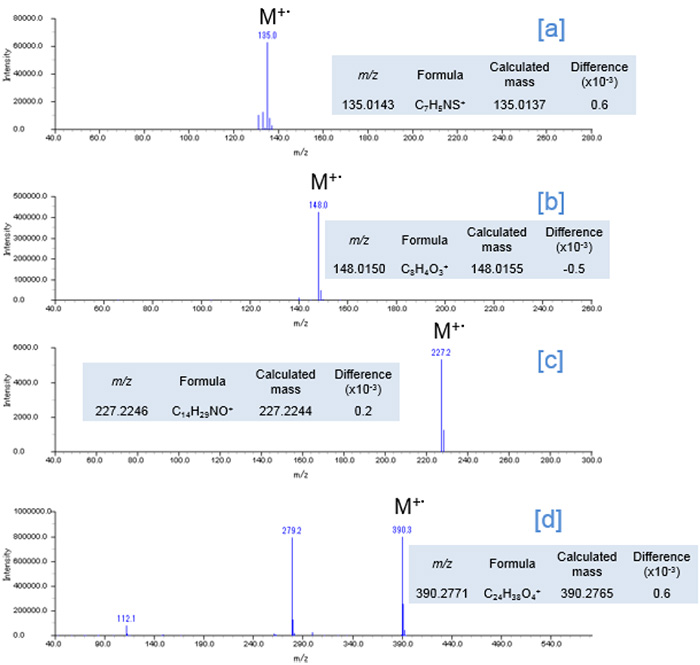
Fig. 4 FI Mass spectra
Results
The 2D Total ion current chromatogram (TICC) maps of the sample by using EI and FI are shown in Figures 1 and 2, respectively. The horizontal axis indicates boiling point separation and the vertical axis shows polarity chromatographic separation. The mass spectra from the region of [a], [b], [c] and [d] on the each 2D maps were shown in Figure 3 and 4, respectively. The compounds eluted at the region from [a] to [d] were assigned as the additives benzothiazole (Fig. 3 [a]), phthalic anhydride (Fig. 3 [b]), N,N-dimethyl dodecanamide (Fig. 3 [c]) and diisooctyl phthalate (Fig. 3 [d]) in the results by using a NIST library search of the EI spectra. It should be noted here that when a single GC column is used, the identification of benzothiazole is very difficult because this compound completely coelutes with an NBR thermal decomposition compound. To further complicate this situation, the abundance of this compound is very low relative to the interfering thermal decomposition product. However, the benzothiazole was easily separated chromatographically by the addition of a 2nd column (GCxGC conditions). The EI spectrum of diisooctyl phthalate showed only fragment ions (Fig. 3[d]). On the other hand, the FI spectrum showed the molecular ion with a relatively high intensity (Fig. 4[d]) which allowed for the estimation of its elemental composition. The composition result was consistent with the library search result by EI. Furthermore, the results of the elemental composition estimations for the fragment ions by EI were also consistent with the assigned composition of the molecular ion.
Conclusion
The additives such as benzothiazole in the polymer material were easily separated chromatographically by the GCxGC technique. Verification of library search of the EI mass spectra and the elemental composition estimation for the molecular ion by FI mass spectra were effective for the qualitative identification of the additives. Reliable qualitative analysis for the additives in the polymer material is confirmed by PY/GCxGC/HRTOFMS.
- Please see the PDF file for the additional information.
Another window opens when you click. 
PDF 1,194.8KB
Related Products
Are you a medical professional or personnel engaged in medical care?
No
Please be reminded that these pages are not intended to provide the general public with information about the products.
