Wako Pure Chemical Industries,Ltd. Reagent Research Laboratories (Tokyo)
In June 1922, Takeda Pure Chemicals Ltd. was devolved from the Chemicals Department of Takeda Chobei Shoten (currently Takeda Pharmaceutical Company Ltd.) and established as a separate company. In 1947, the company name was changed to Wako Pure Chemical Industries, Ltd., the name that is still in use.
Becoming the strength for next-generation science
Wako Pure Chemical Industries has been conducting business based on the management philosophy established at the foundation of the company,“Promote the development of science, technology and academic research, to contribute to enriching the lives of people.” Employees always carry small cards with the management philosophy and a brief outline of action guidelines, and refer to them daily. The three main divisions of Laboratory Chemicals, Speciality Chemicals, and Clinical Diagnostic Reagents support the needs of researchers, medical personnel and a wide range of industries.
Clinical Diagnostic Reagent research laboratories are located in Tokyo and Osaka. In a system coordinating research and development, including synthesis, purification and solution preparation of reagents, it meets the needs of customers rapidly and accurately. Cutting-edge technology based on synthetic organic chemistry techniques have been introduced and perfected over many years. Developments for reagents include environmental analysis, genetics and cellular organisms, chromatography, and organic reagents.
Wako Pure Chemical Industries began using NMR in order to find a highly precise analytical method to ensure reagent quality. In particular, the preparation of standards is time-consuming and expensive and in recent years there has been an increased demand to ensure the reliability of the analyses. In this situation, NMR (qNMR) and stability tests have ensured world class quality that is also cost-effective.
The NMR instruments are managed by an administrator and operated only by qualified users after a training course on use of the instruments, a competency assessment is conducted to ensure that only qualified users are allowed to use qNMR.
At the Clinical Diagnostic Reagent research laboratories in Tokyo, there is an JNM-ECA600 NMR system and JNM-ECS400 NMR systems, all manufactured by JEOL RESONANCE. The 600 MHz system is sed mainly for research and development, whilst the 400 MHz systems are primarily at the manufacturing sites, so the systems are strategically located according to the requirement. The high performance of the 600 MHz system enables analysis on small samples thus enabling research and development to be performed with even greater efficiency.
In 2013, the Tokyo plant was the first facility in Japan to receive accreditation for their test method for quantitative NMR for conformance to the ISO/IEC17025 International Standard for general requirements for the competence of testing and calibration laboratories.
Wako Pure Chemical Industries is hoping to expand the use of product quality assurance based on qNMR and business overseas. “As an R&D-oriented company, we aim to partner innovation by our customers thus providing the added value that exceeds customer expectations, so that this kind of impressive research result can be obtained.”

Keiji Oono, Director of the Reagent Research Laboratories, Wako Pure Chemical Industries, Ltd.

Reagent Research Laboratories (part of the Tokyo plant)
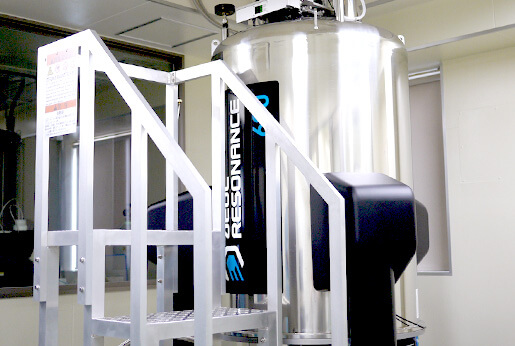
JNM-ECA600 NMR system
